Assessing CO2 storage mechanisms in marine shallow sediments to mitigate leakage risks from sub-seabed carbon storage: a numerical simulation study
为评估离岸高盐水层CO₂封存若发生泄漏对海底浅层沉积物环境的影响,本文以南海恩平15-1示范工程为背景,构建二维剖面概念模型,采用CMG-GEM模拟CO₂-水-岩反应与多相运移。计算表明:
- 一旦CO₂通过断层/盖层裂隙进入海底0–100 m浅层沉积层,70 %以上以溶解相被快速固定,残余气相与碳酸盐沉淀各占约10–20 %。
- CO₂溶解导致孔隙水酸化(pH最低4.4),促进斜长石、钾长石溶蚀,并沉淀方解石、白云石、道森石等次生碳酸盐。
- 存在“临界泄漏速率”——单点>0.2 m³/d、多点>0.3 m³/d时,早期沉淀的碳酸盐会再次被酸液溶蚀,CO₂突破至海床风险显著增加;低于该阈值,沉积物可长期(100 a)封存泄漏CO₂。
- 多点低流速泄漏因分散酸化前缘,可提高20 %左右矿物固定量,为工程上“化整为零”降低环境风险提供理论依据。研究首次给出了南海典型砂-粉砂互层沉积物的速率安全上限,为海底CCUS监测方案与法规制定提供了量化指标。
CMG软件应用情况
- 模拟平台:CMG-GEM(v2023)组分-地球化学耦合模拟器;状态方程采用Peng-Robinson与Soave-Redlich-Kwong双模型。
- 前处理:用CMG-WinProp建立CO₂-CH₄-海水体系PVT与溶解度表,结合Henry定律校正低压(<2 MPa)下的CO₂溶解度。
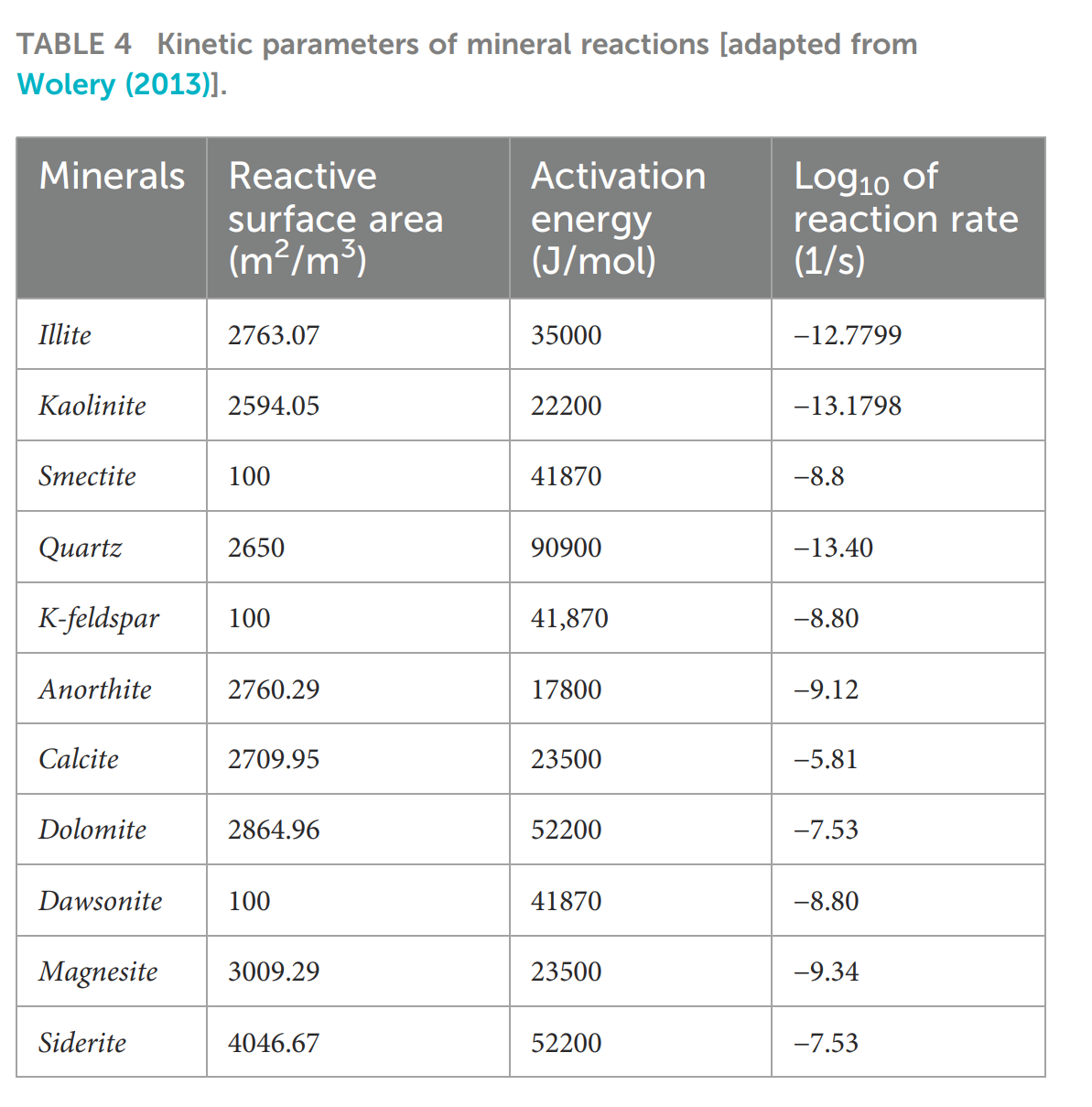
- 地球化学:内嵌Wolery数据库,定义14种矿物(石英、斜长石、钾长石、伊利石、高岭石、蒙脱石、方解石、白云石、菱铁矿、菱镁矿、道森石等)的溶解/沉淀动力学参数;反应速率按Bethke方程计算,并随矿物摩尔数动态更新反应面积。
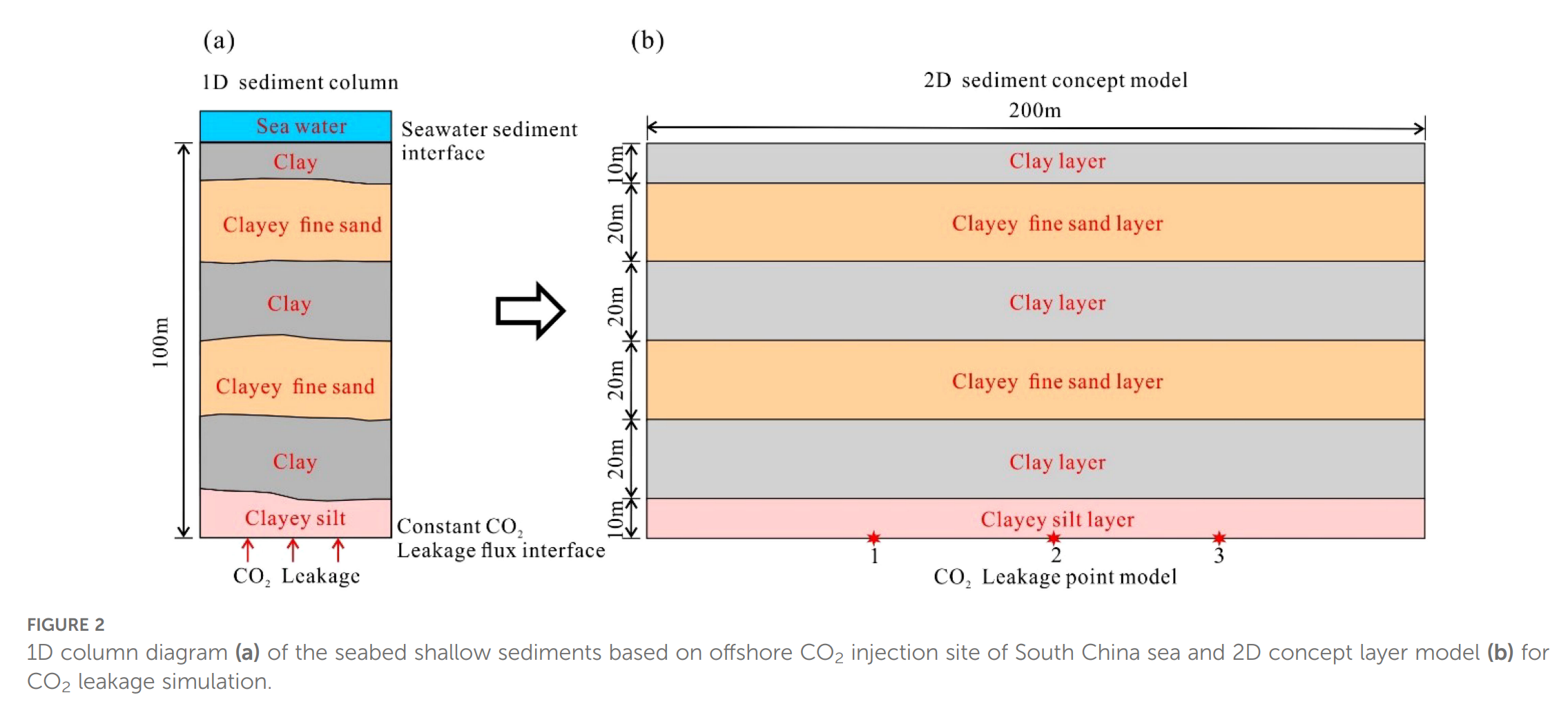
- 网格:2-D剖面200 m×100 m,2 m×2 m均匀网格,共5000个单元;垂向划分泥-粉砂-砂互层三层,分别赋予不同渗透率与孔隙度。
- 模拟方案:18组(单点9组+多点9组),泄漏速率0.005–0.5 m³/d,模拟期100 a;通过质量平衡、矿物摩尔变化、pH场等评估封存贡献比例与临界速率。
- 结果验证:与南海现场孔隙水化学、STEMM-CCS原位释放试验及多家实验室柱实验对比,CO₂溶解比例、酸化前锋形态与矿物变化趋势一致。
成都理工大学能源学院(油气藏地质与开发国家重点实验室)


Abstract
The geological storage of CO2 in offshore saline aquifers has been implemented at various sites worldwide. While subsea sediments can serve as a critical barrier against potential leakage from deep storage formations, the mechanisms and storage capacity for CO2 trapping within these sediments remain inadequately understood. This study developed a two-dimensional conceptual model of shallow sediments based on the Enping15–1 Carbon Capture and Storage (CCS) project site in the South China Sea. Furthermore, the CO2-water-rock reaction and storage mechanism were simulated using CMG-GEM, and the process of CO2 leakage into the sediments was investigated. The results indicate that CO2 leakage into the shallow seabed sediments is primarily sequestered through dissolution in pore water, accounting for 70% of the total sequestration, while residual and mineral trapping contribute 10–20%. The dissolution of CO2 leads to pore water acidification, which triggers the dissolution of anorthite and K-feldspar under the prevailing initial geochemical conditions. Dynamic reaction behavior is mainly observed at the leading edge of the acidified plume. However, if the leakage rate exceeds a critical threshold, the advancing acidified plume front causes partial dissolution of previously precipitated carbonate minerals. The critical leakage rate is determined to be 0.2 m³/day for single-point leakage and 0.3 m³/day for multi-point leakage. Notably, multi-point, low-velocity leakage enhances secondary storage within the sediment, thereby reducing the risk of CO2 release into the overlying seawater.
