Effects of permeability, grid resolution, and carbonate reactions on CO2 distribution and sulphate scaling in seawater-flooded carbonates
本文针对海水驱油过程中常见的结垢问题,系统研究了CO₂在油、水相间的分配及其对碳酸盐与硫酸盐结垢风险的影响。通过构建三维三相数值模型,模拟了不同温度、压力、离子浓度和CO₂含量条件下,方解石溶解与富镁碳酸盐(如白云石)沉淀之间的耦合关系。研究发现,CO₂从残余油相向注入水中的转移显著改变了水相pH值,进而影响钙、镁、钡、锶、硫酸根和碳酸氢根等关键离子浓度,最终影响生产井的结垢风险。此外,渗透率分布对CO₂可利用性、温度传播及结垢离子运移具有显著影响。研究采用CMG GEM模拟器实现了多相流动与地球化学反应的耦合模拟,强调了在碳酸盐岩油藏中,管理温度、离子浓度与CO₂分布对于控制结垢、保障采收率的重要性。
CMG软件应用情况:
本研究使用加拿大CMG公司开发的GEM组分-地球化学耦合模拟器,构建了一个三维三相四分之一五点井网模型,模拟了水驱过程中油、水、气三相流动与矿物反应之间的相互作用。模型中考虑了11种水相离子组分(如Ca²⁺、Mg²⁺、Ba²⁺、SO₄²⁻、HCO₃⁻等)与5种矿物反应(方解石、白云石、硬石膏、石膏、重晶石等),并引入了温度依赖的溶解度模型与Pitzer活度模型,实现了CO₂分配、pH演化与结垢风险动态预测。
研究结论:
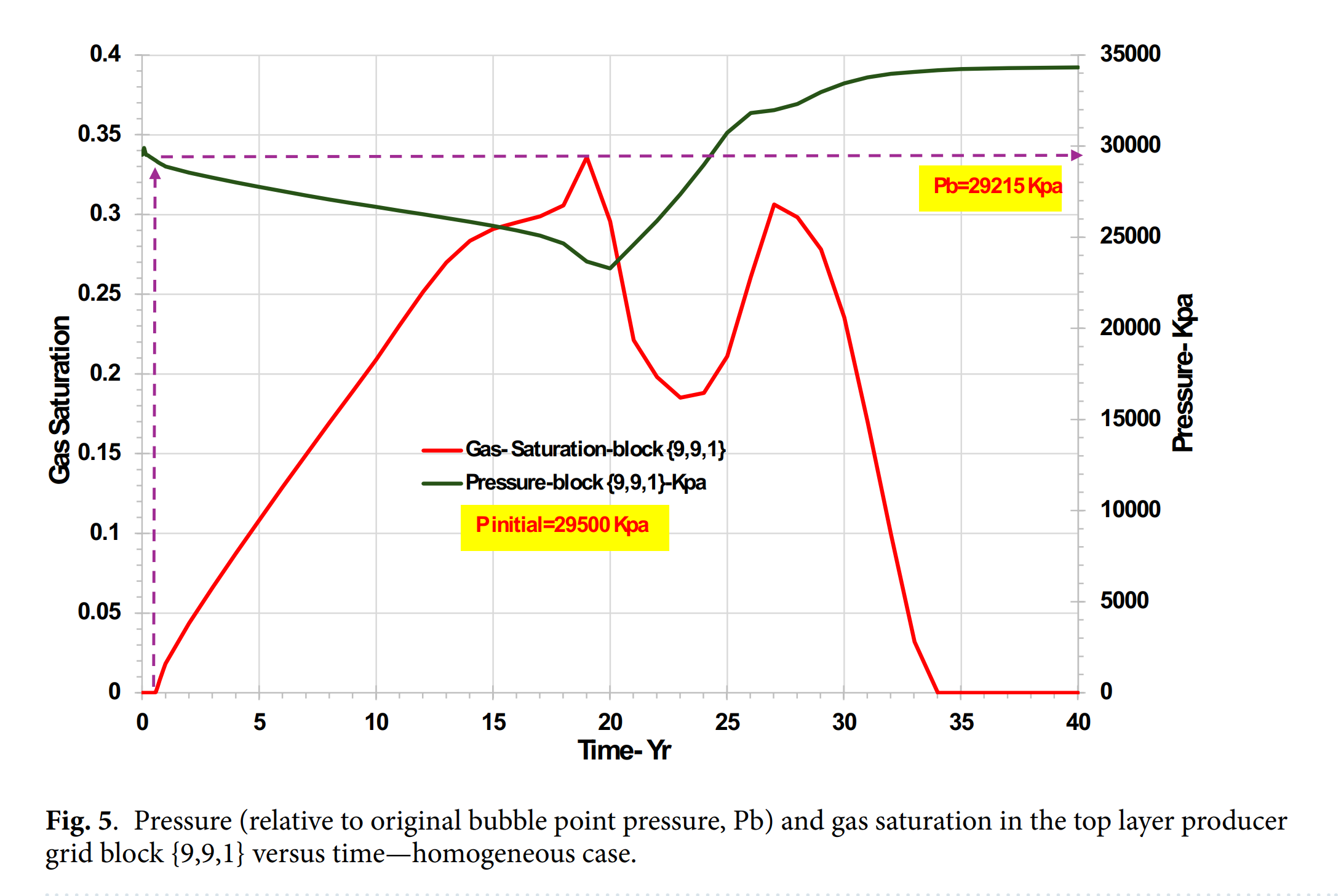
- CO₂从油相向水相的分配行为是控制碳酸盐结垢的关键机制,显著影响pH值与矿物溶解/沉淀平衡。
- 方解石溶解与白云石沉淀之间存在明确化学计量关系:每沉淀1摩尔白云石,需溶解2摩尔方解石,释放出1摩尔钙离子,可用于后续硬石膏或重晶石反应。
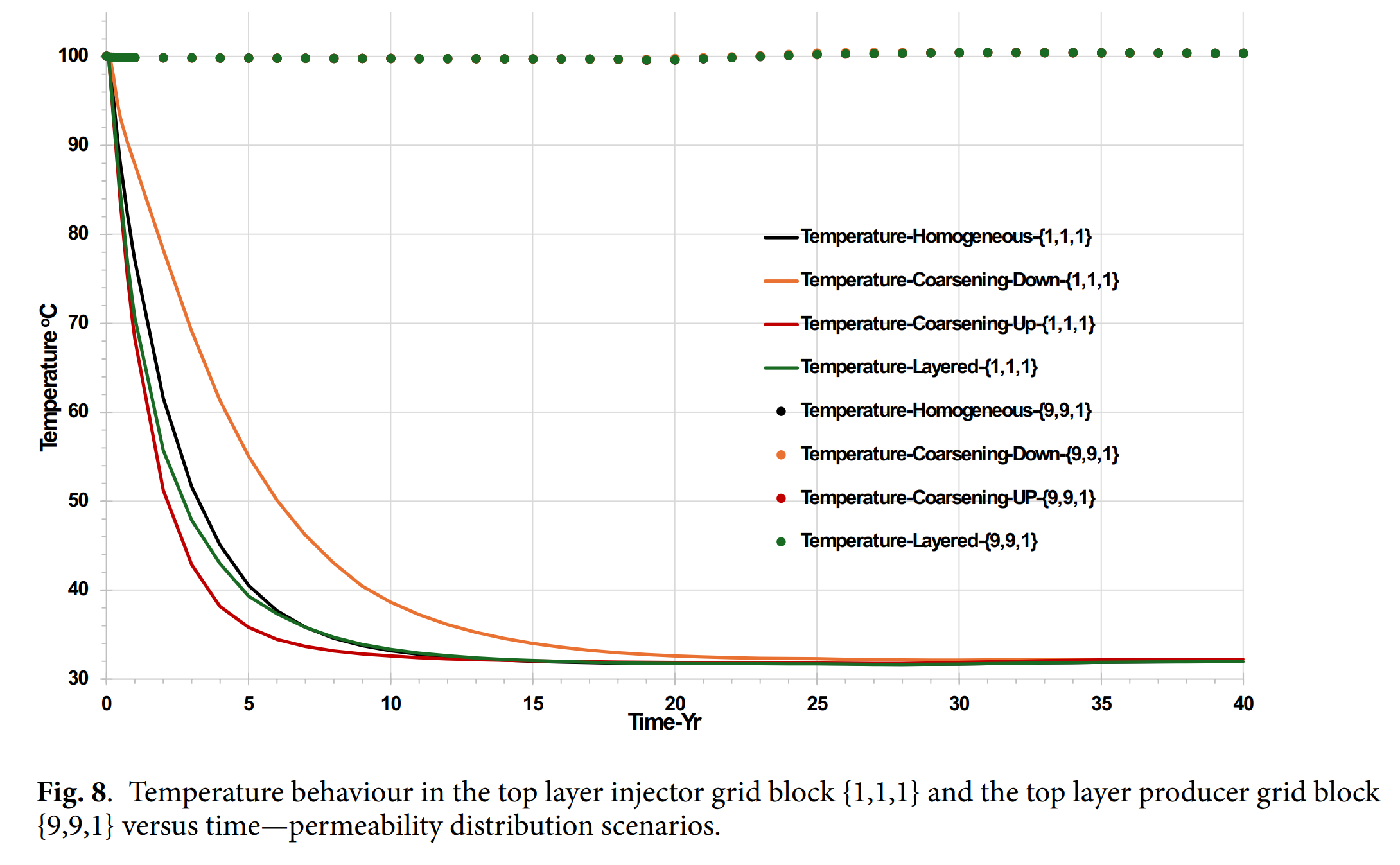
- 渗透率分布显著影响CO₂可利用性、冷却前沿传播速度及结垢离子突破时间,异质模型中结垢风险更高且持续时间更长。
- 网格分辨率对结垢预测结果影响有限,但粗网格会高估混合程度,导致硫酸盐突破时间被延迟约10年。
- 储层本身可通过矿物反应自然消耗部分硫酸根离子,从而延迟生产井结垢风险,可能降低对注入水脱硫处理(LSSW)的依赖。
作者单位:
- 英国赫瑞-瓦特大学(Heriot-Watt University)能源、地球科学、基础设施与社会学院
Abstract
During oilfield production, mineral scale deposition in production wells and surface facilities presents major difficulties, particularly when water breakthrough occurs. Hard sulphate scales, like barite, are frequently the result of incompatible interactions between the formation and injection waters. In contrast, carbonate scales are caused by variations in the temperature, pressure, pH, composition of the brine, and the amounts of CO2 in the aqueous and hydrocarbon phases. In waterflooded reservoirs where injection water composition can be controlled, this study investigates the effects of temperature, ionic concentration, pH, and CO2 availability on the risks of carbonate and sulphate scaling. Particularly, scaling hazards in carbonate-rich formations are greatly influenced by precipitation of magnesium-rich carbonate. The significance that CO2 partitioning from hydrocarbons into injected saltwater plays in scaling estimates has been ignored in earlier studies. To close this gap, this study investigates how temperature and reservoir pressure affect oil recovery and scale management, particularly in systems that dip below bubble point pressure. A commercial reservoir simulator, which couples aqueous and mineral geochemistry with three-phase fluid flow calculations, has been used in this study. Equilibrium reactions have been considered in three-dimensional (3D) models. Oilfield data have been used to identify the parameters of significance to consider in the calculations, such as ionic concentrations, hydrocarbon composition, mineral components etc. Key findings, importantly, the results identify that for the reservoir temperature of 100 °C considered and for the primary mineral assemblage, calcite dissolution and magnesium-rich carbonate precipitation are interdependent. They are affected by the abundance of CO2 in the residual oil phase, and this evolves over time, impacting the concentration of calcium and magnesium in the brines traversing the reservoir. Temperature changes around the injection wellbore also impact component and mineral solubilities, especially in terms of anhydrite and gypsum reactions. All these factors impact the calcium, magnesium, barium, strontium, sulphate and bicarbonate concentrations at the production well, and hence the scaling risk in the production system. In conclusion, managing reservoir conditions such as temperature, ionic concentrations, and CO2 distribution is important for reducing the likelihood of scale deposition while preserving oil recovery in carbonate-rich, water-flooded reservoirs.
