Effects of pressure depletion rate, solvent, and surfactant on non-equilibrium reactions in foamy oil
本研究采用可视化方法探讨了重油系统中泡沫油的行为。设计了一个Hele-Shaw网格,用于观察系统压力降低时泡沫油的体积膨胀。这种方法便于检查泡沫油在压力衰减下的界面演变,并分析气泡的大小及其分布。通过Minitab软件规划了15次实验,旨在观察泡沫油过程中气泡的分布及其稳定性。研究还扩展到研究表面活性剂、溶剂类型和压力降低率对泡沫油的影响。结果表明,高浓度的表面活性剂、高百分比的CO₂溶剂和快速的压力下降率都有助于生成微气泡,并增强泡沫油的体积膨胀和稳定性。然而,根据进行的能量分析,建议采用较低的压力降低率。最后,将15次实验的条件应用于CMG,以得出两个非平衡反应,分别用于气泡的生成和破灭。反应速率与过程中的压力降低率和泡沫油中气泡抵抗破灭的表面活性剂浓度相关。
CMG软件应用情况:
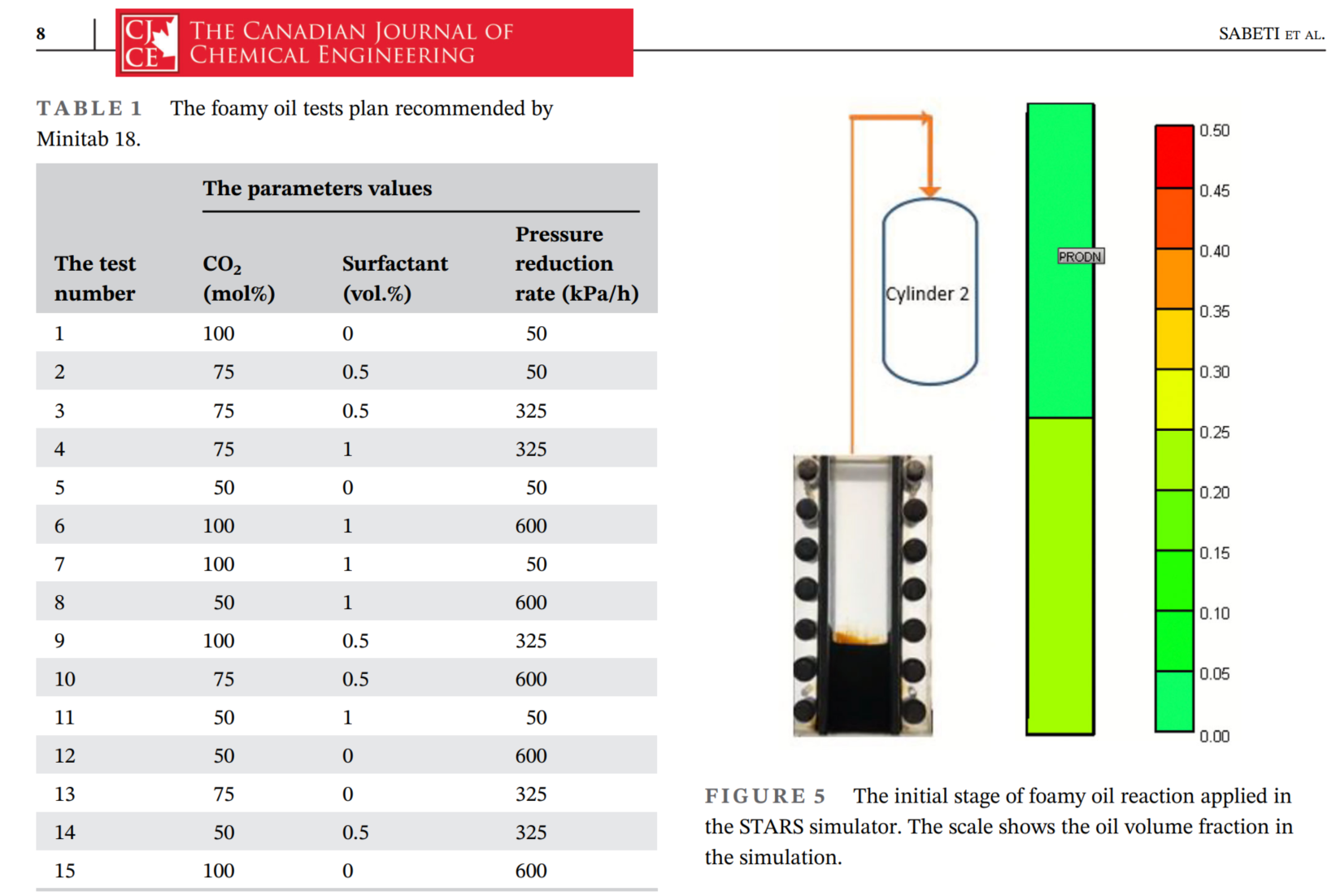
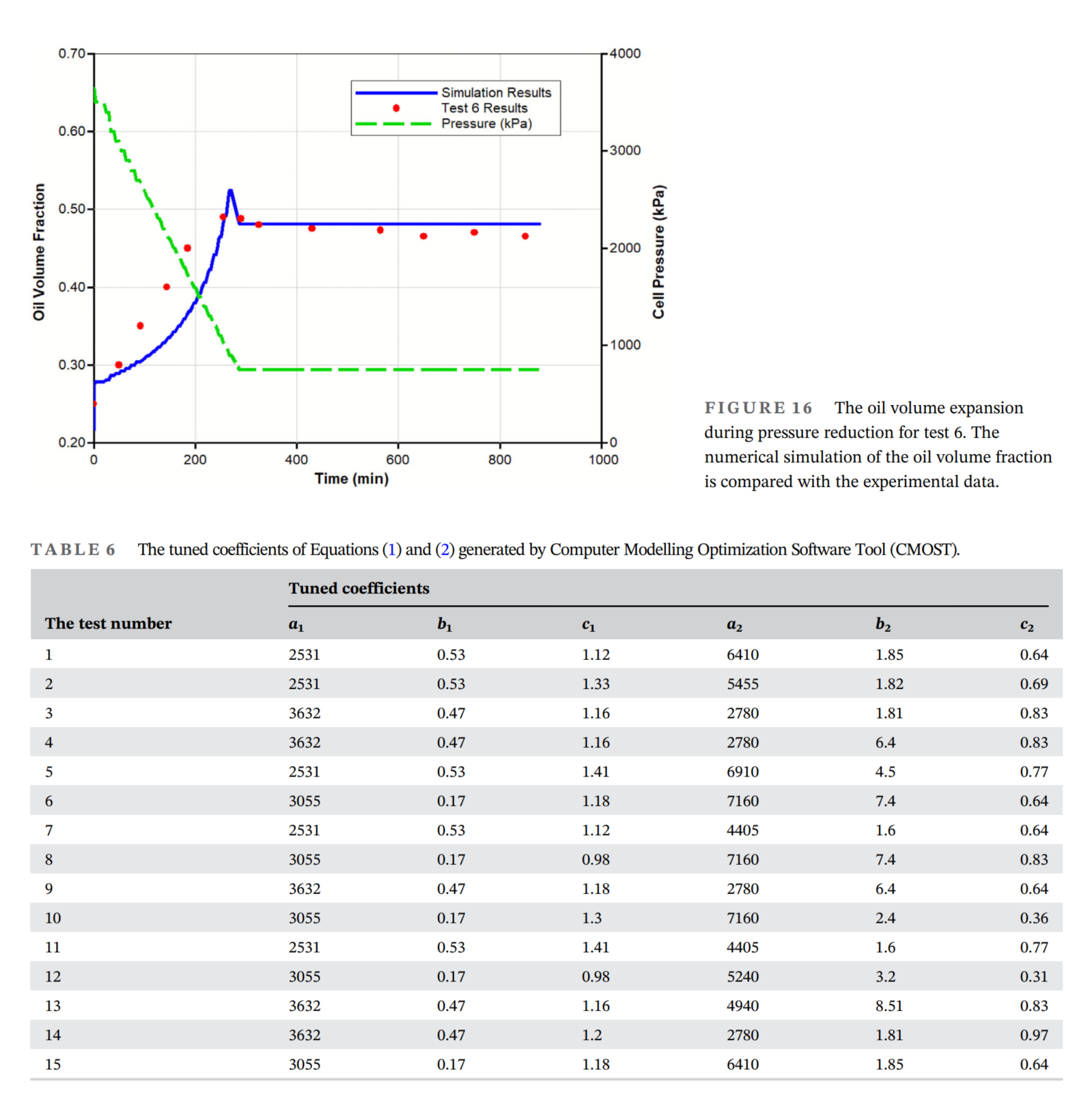
在本研究中,使用了CMG(Computer Modelling Group)公司的STARS模拟器来模拟泡沫油的体积膨胀。Hele-Shaw网格在CMG软件的STARS模块中进行了模拟。定义了两个垂直的网格:底部一个用于Hele-Shaw网格内的区域,第二个网格用于存储气体的气缸2。由于实验在Hele-Shaw网格中以批量相进行,因此两个网格都被视为具有100%的孔隙度。相对渗透率被定义为只有气相可以通过两个网格的边界。底部网格的面积和体积与Hele-Shaw网格相同。初始油体积分数在Hele-Shaw网格中计算为0.25,并被视为第一个网格的初始油饱和度。第二个网格的油体积分数设置为零,因为它仅包含存储的气体。通过恒组成膨胀和差异分离实验的油和溶剂的物理性质(如油粘度、平衡条件、密度和分子量)被应用于STARS模块。在模拟器中定义了两个主要反应:一个与泡沫油中气泡的生成相关,另一个与气泡的破灭相关。这些反应代表了本研究中进行的模拟中发生的非平衡反应。通过CMG模块CMOST(Computer Modelling Optimization Software Tool)对这些反应中的未知系数进行了优化,以最小化目标函数并提高模拟的准确性。
结论:
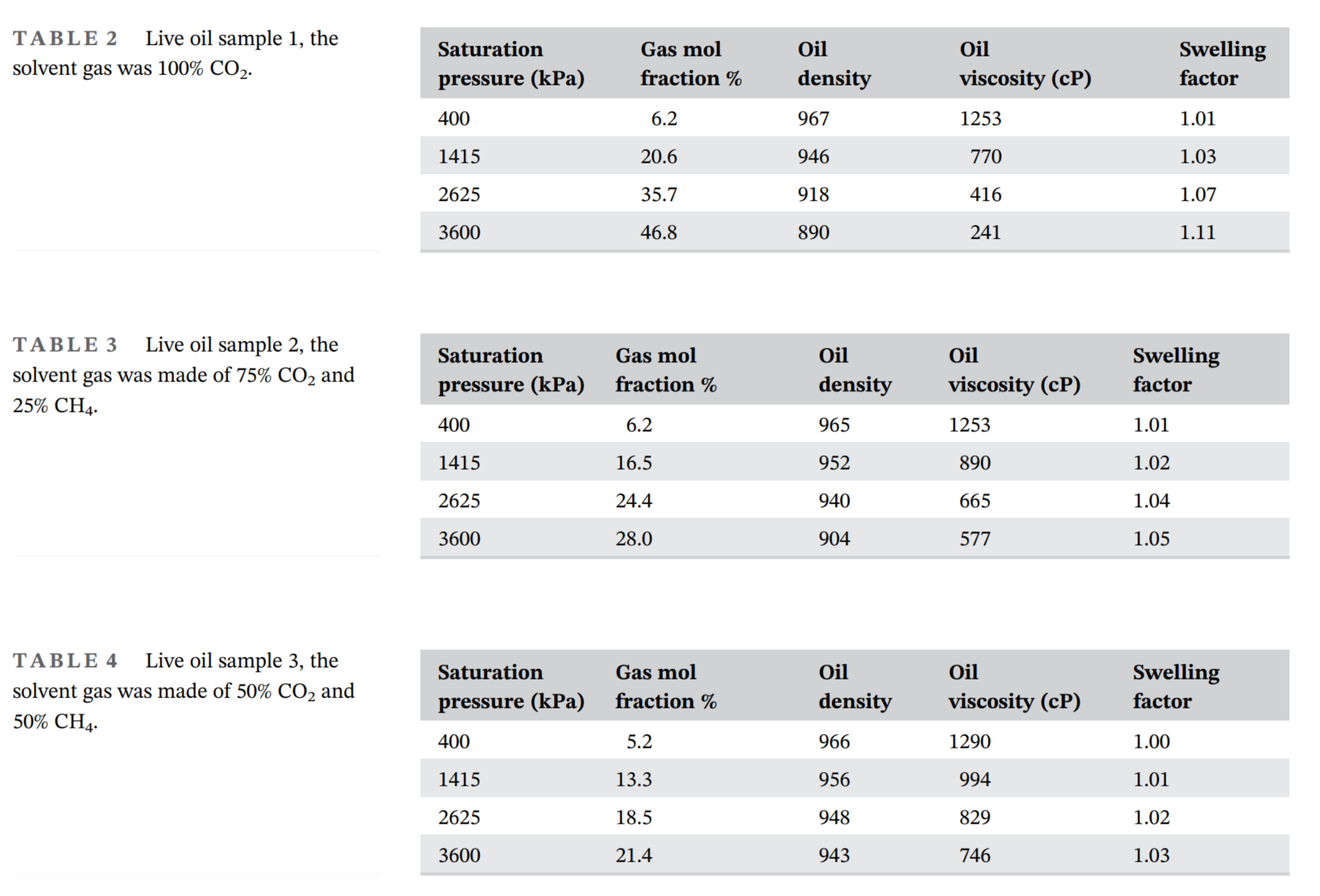
- 通过差异分离和恒组成膨胀测试,确定了三种不同溶剂制备的活油样本的基本属性,包括溶解气含量、密度和粘度。
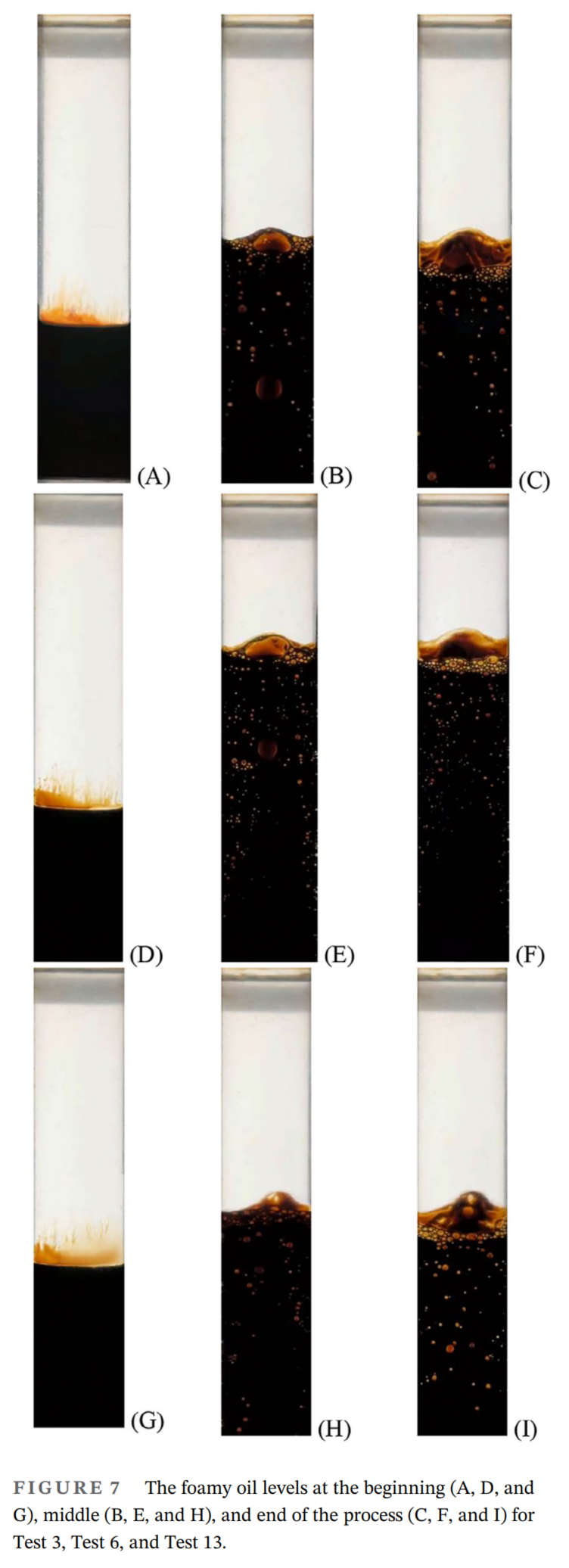
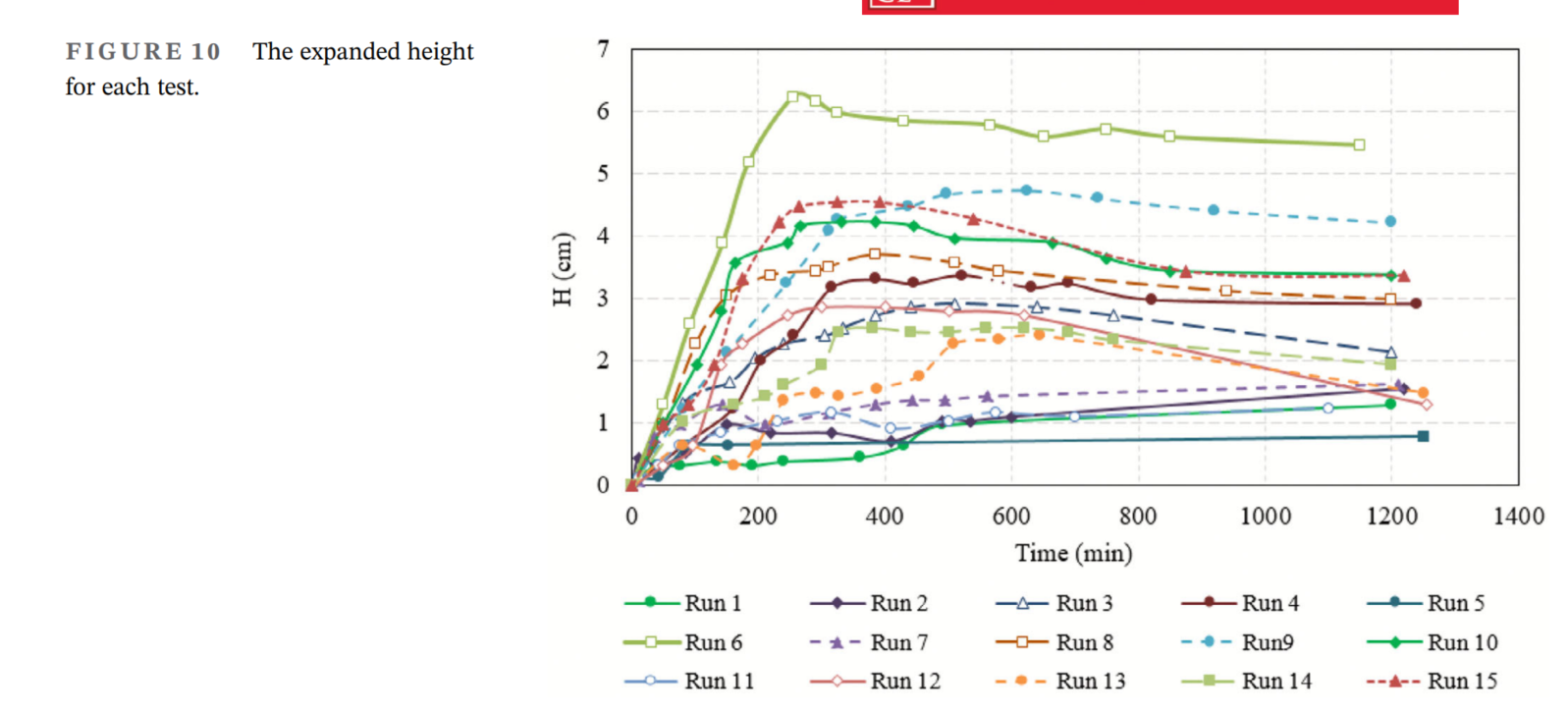
- 泡沫油行为受溶剂类型、表面活性剂浓度和压力降低率的影响显著。高浓度的CO₂溶剂(100%摩尔分数)、大量的表面活性剂(重油体积的1%)和快速的压力降低率(600 kPa/h)可实现最高的泡沫油稳定性和体积膨胀。
- 通过Minitab软件确定了实现最大泡沫油稳定性和最小能耗的最佳条件:100% CO₂溶剂浓度、0.75%表面活性剂浓度和356 kPa/h的压力降低率。
- 通过定义两个非平衡反应(气泡生成和破灭),并结合压力降低率、表面活性剂浓度和溶剂气中的CO₂浓度等变量,模拟结果表明这些反应可以用来估算泡沫油随时间的体积膨胀。
作者单位:
加拿大里贾纳大学工程与应用科学学院石油系统工程系。
Abstract
This research employed a visual method to explore the behaviour of foamy oil in heavy oil systems. A Hele-Shaw cell was designed for observing the volumetric expansion of foamy oil as the system pressure decreased. This approach facilitated an examination of foamy oil’s interface evolution under pressure depletion and an analysis of bubble sizes and their distribution. Using Minitab, 15 experiments were strategized, aimed at observing the distribution of bubbles and their stability during the foamy oil process. The investigation also extended to studying the influence of surfactants, solvent type, and pressure reduction rate on foamy oil. The findings suggest that a high concentration of surfactant, a high percentage of CO2 solvent, and a rapid pressure drop rate all contributed to the generation of microbubbles and enhanced volumetric expansion and stability of foamy oil. However, in light of the conducted energy analysis, a lower rate of pressure reduction is recommended. Finally, the conditions of the 15 experiments were applied to the CMG to derive two non-equilibrium reactions for bubble generation and collapsing. The reaction rates are such that they relate bubble generation to the pressure reduction rate of the process and bubble resistance to collapsing to the surfactant concentration of the foamy oil.
