MODELING THE EFFECTS OF SALT PRECIPITATION & KINETIC MINERAL REACTION ON WELL INJECTIVITY DUE TO CARBON DIOXIDE INJECTION IN DEEP SALINE AQUIFERS
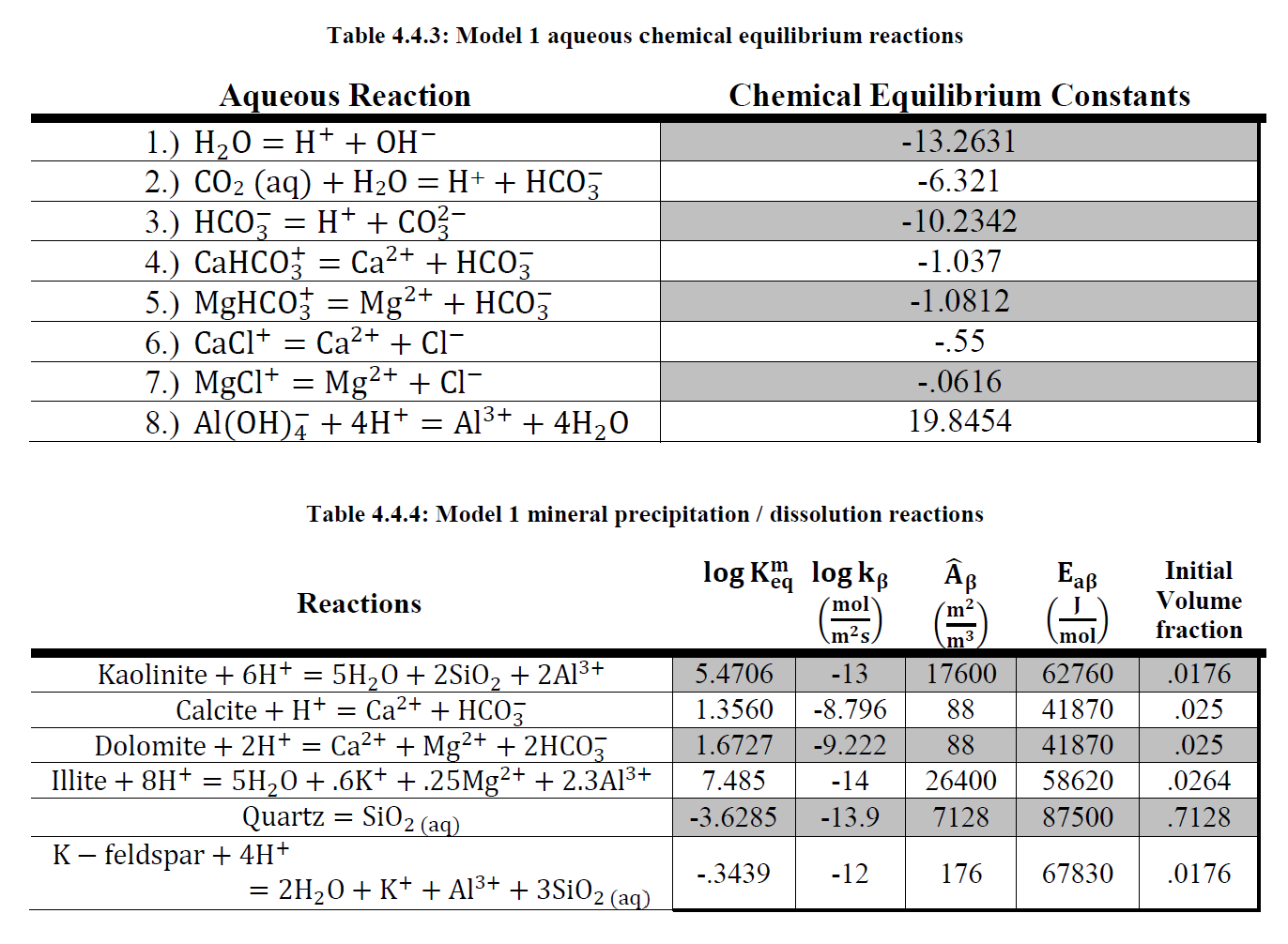
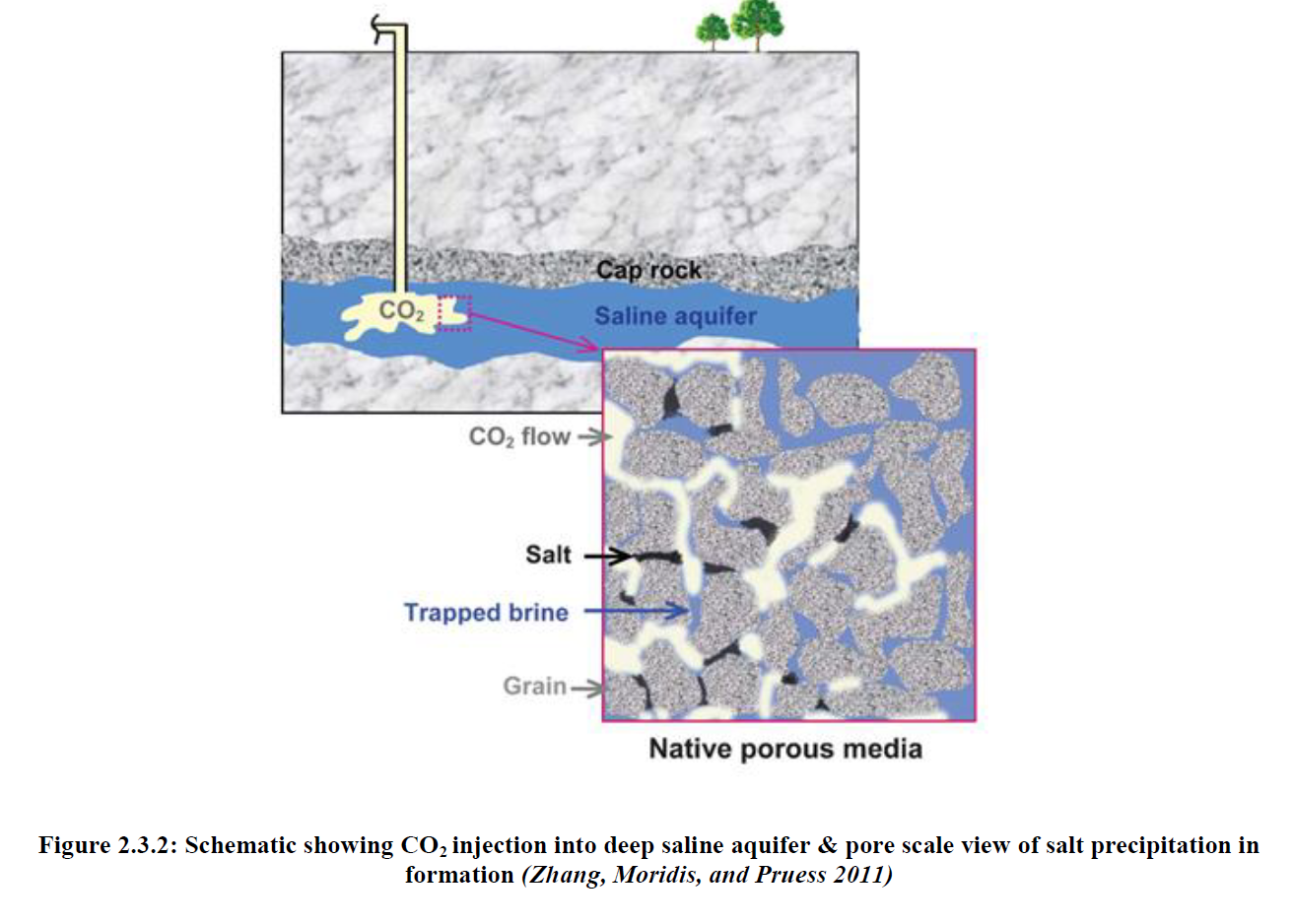
Within recent years public interest has steadily been growing over global climate change and the lasting effects caused by greenhouse gas emissions, carbon dioxide in particular. In attempting to reduce CO2 emissions and mitigate the associated negative effects, carbon dioxide capture and storage, also known as carbon dioxide sequestration, has emerged as a method by which CO2 emissions may effectively be reduced long term. Of the potential geologic storage sites for captured CO2, deep saline aquifers present the most attractive storage option. Unfortunately, little is known about the complex processes which take place between CO2, the host formation, and in situ brine at the conditions found within these aquifers. Mineral dissolution and precipitation reactions which take place in response to the acidic environment formed once CO2 is injected have the potential to alter formation properties. In addition, Halite precipitation due to vaporization of the brine phase into the continuous flowing dry CO2 phase is a phenomenon which has been observed extensively on the experimental scale and has been observed to negatively impact sample porosity and permeability.
The impacts of these geochemical mineral reactions on formation properties and well injectivity on the field scale is however debated within industry and thus a field scale simulation study was performed in order to evaluate and quantify the potential significance. The commercial compositional reservoir simulation package GEM was used to develop multiple models in order to simulate prolonged CO2 injection into a siliciclastic deep saline aquifer formation. The scale and extent of host mineral dissolution and precipitation as well as halite mineral precipitation following CO2 injection were observed, and the impacts to well injectivity assessed. A sensitivity study was performed in order to assess the potential impacts of CO2 injection rate, formation temperature, and formation salinity on halite precipitation and its negative effects. In addition the potential for mitigation of the negative effects of halite precipitation through means of a fresh water pre flush was studied.
Following CO2 injection, dissolution was observed to be the predominant mineral reaction however the limited scale of dissolution resulted in minimal impact on well injectivity. The extent of halite precipitation was limited to the near well region with limited impacts on well injectivity. Formation salinity appears to be the most significant property in impacting halite precipitation with noticeable impacts to formation porosity and permeability at higher salinities. These effects can however be effectively negated with a 3 month fresh water injection period prior to the onset of CO2 injection.

13
Chapter 2.2 CO2 /Brine/ Mineral properties within Deep Saline Aquifers
One cannot begin to fully understand the complex processes which govern CO2 mobility and storage in deep saline aquifer systems and address the challenges to prolonged injection without first considering the conditions under which CO2 exists in such environments and acknowledging the physical and chemical processes active. At normal atmospheric conditions representing standard surface temperature and pressure CO2 is found as a thermodynamically stable gas heavier than air. The phase diagram of pure CO2 shows a critical temperature of 31°C and a critical pressure of 7.38 MPa. Below this temperature and/or pressure the CO2 is either in a liquid or vapor phase, as can be seen in Figure 2.2.1 below.
Chapter 4.1 CMG GEM Model
In this work the fully coupled geochemical compositional Equation of State reservoir simulation software GEM developed by CMG was used to model CO2 injection into a saline aquifer. The commercial software designed to better model compositional and unconventional reservoir types is capable of modeling complex processes such as gas condensates, volatile oils, gas cycling, Water-Alternating-Gas (WAG) processes, and many other multi-component reservoir scenarios and is also capable of model gas injection processes such as miscible floods, and vaporizing or condensing gas drives. The simulator is used extensively in gas injection scenarios as it is effective in simulating all the important mechanisms of a miscible gas injection process i.e. vaporization and swelling of oil, condensation of gas, viscosity and interfacial tension reduction, and the formation of a miscible solvent bank through multiple contacts. The GEM model developed is used to model the following phenomena
1. Convective and dispersive flow in porous media
2. Phase equilibrium between gas and aqueous brine phase
3. Chemical equilibrium for reactions between aqueous components
4. Mineral dissolution and precipitation kinetics
5. Adjustments in well injectivity due to modification of reservoir characteristics
The GEM simulator can be in explicit, fully implicit and adaptive implicit modes to solve complex partial differential equations. In a majority of cases, only a few grid blocks need to be solved fully implicitly; while most blocks can be solved explicitly. An option exists to select an adaptive implicit method which selects a block’s implicitness dynamically during the computation and can be useful for coning problems where high flow rates can be observed near the wellbore, or in stratified reservoirs with very thin layers. Several other options are provided for selecting implicit treatment.