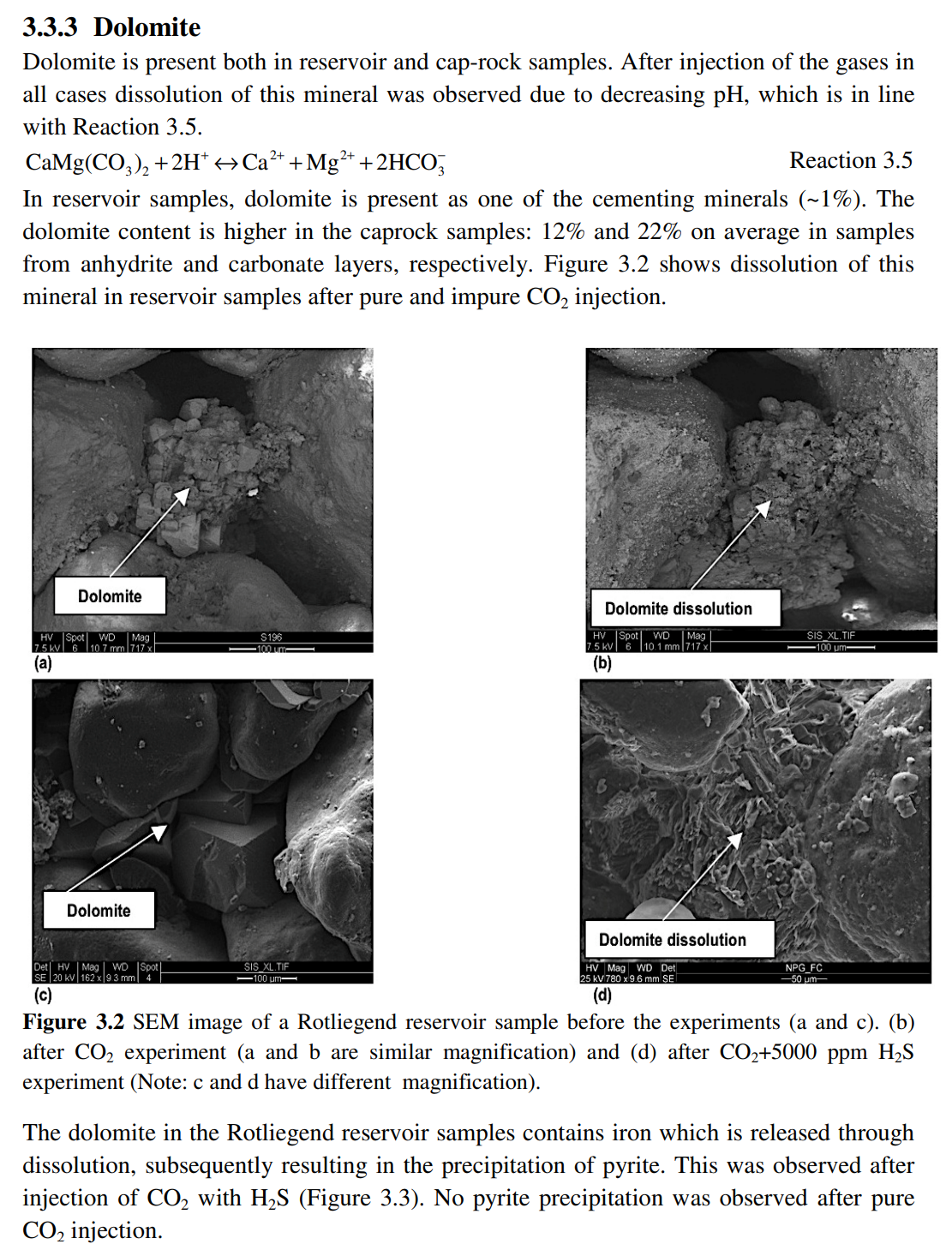
Effects of impurities on subsurface CO2 storage in gas fields in the northeast Netherlands
- 枯竭气田是最有可能进行CO2地下储存的气田之一。由于储层已探明,且封层合格,这些气田在地质时间尺度上保留了气体。然而,与甲烷不同,二氧化碳的注入会由于碳酸的形成而改变盐水的pH值。随后的矿物溶解/沉淀会改变储层和盖层的孔隙度/渗透率。因此,为了充分、安全和有效地储存CO2,需要充分了解地下系统。CO2地下储存的一个重要方面是气体的纯度,这会影响封存过程的风险和成本。为了在实际案例中研究CO2和杂质的影响,我们对荷兰东北部气田的储层和盖层岩芯样品进行了中期(30天)实验室实验(300bar,100°C)。此外,我们试图确定这些油田CO2流体(H2S)中一种可能杂质的最大允许浓度。
-
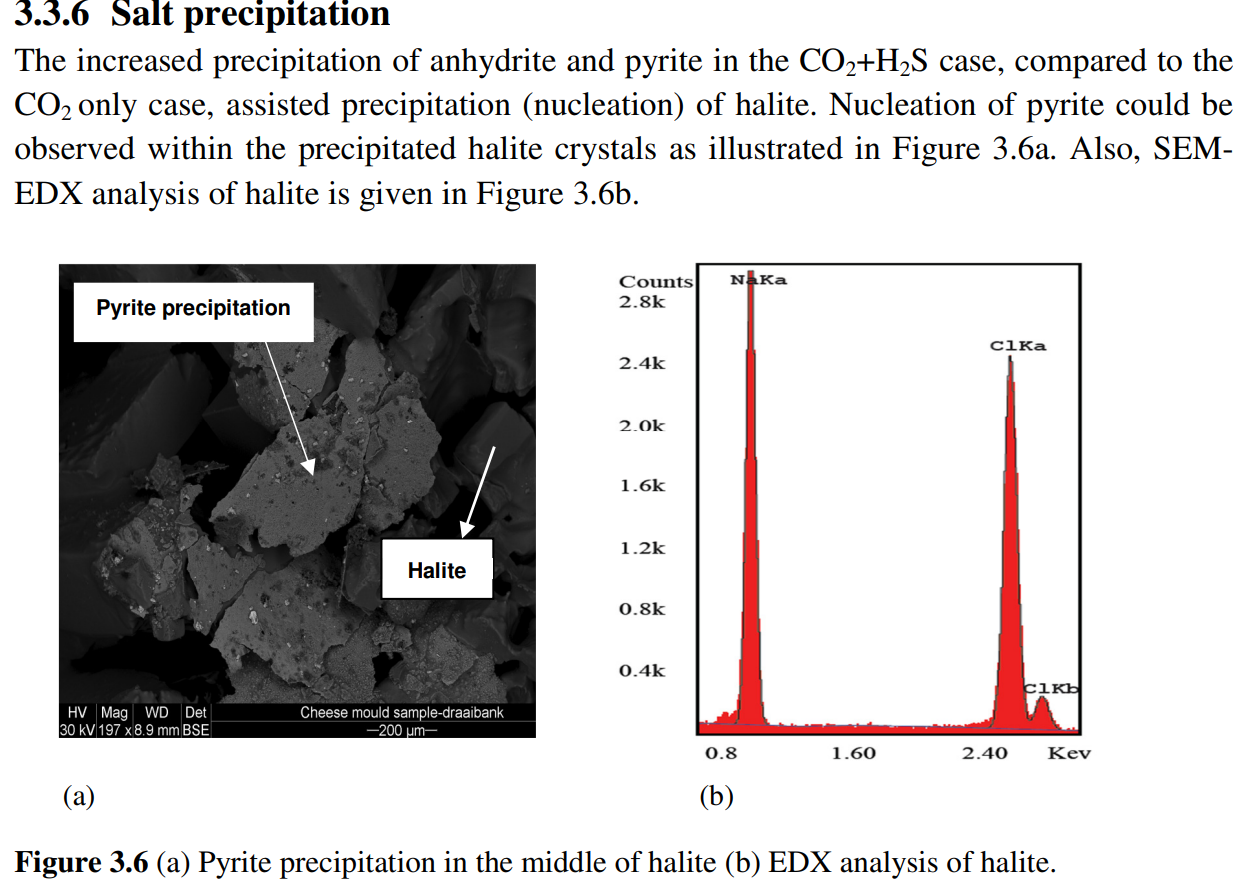
注入的气体为CO2+100 ppm H2S,CO2+5000 ppm H2S与岩芯样品和盐水反应(表2.2)。实验前后,通过扫描电镜(SEM)和X射线衍射(XRD)分析岩芯样品的矿物学变化。此外,还测量了样品的渗透率。实验后,长石、碳酸盐和高岭石的溶解情况如预期。此外,我们还观察到高岭石的新沉淀。然而,在CO2流体中添加H2S时,获得了两个显著的结果。首先,我们观察到硫酸盐矿物(硬石膏和黄铁矿)沉淀。这与纯CO2注入后的结果不同,纯CO2注入时,样品中以硬石膏溶解为主。其次,H2S存在时会发生严重的盐沉淀。这主要是由于硬石膏和黄铁矿的成核作用导致了盐沉淀,在较小程度上是由于H2S在水中的溶解度较高以及H2S存在时气相的含水量较高。这一点已通过使用CMG-GEM(CMG,2011)软件模拟得到证实。
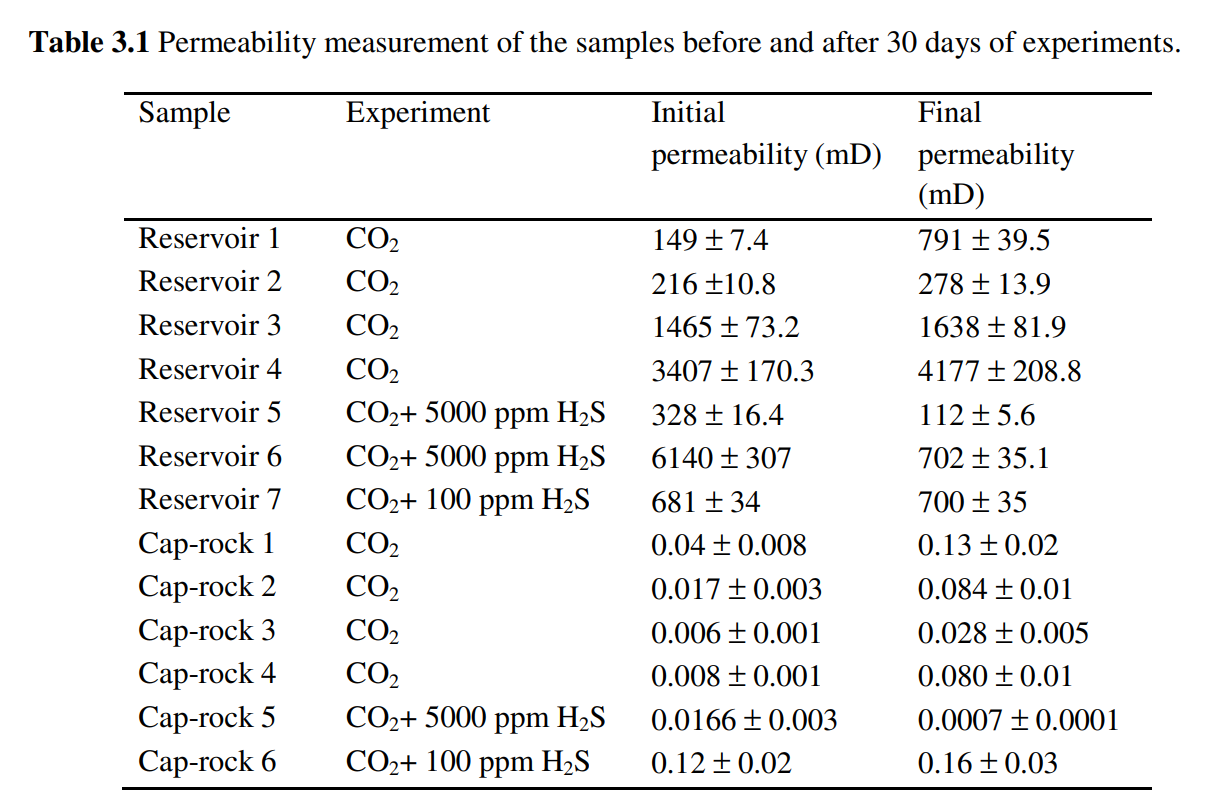
- 石盐、硬石膏和黄铁矿的沉淀以不同的方式影响样品的渗透率。注入CO2+100 ppm H2S后,储层样品的渗透率增加了≤3%. 盖层样品的渗透率增加了1.3。然而,在添加5000 ppm H2S后,所有样品的渗透率都显著下降。在CO2+100 ppm H2S的情况下,石盐、硬石膏和黄铁矿沉淀确实平衡了矿物溶解,使样品的渗透率变化最小。
Abstract
- Depleted gas fields are amongst the most probable candidates for subsurface storage of CO2. With proven reservoir and qualified seal these fields have retained gas over geological time scales. However, unlike methane, injection of CO2 changes the pH of the brine due to formation of carbonic acid. Subsequent dissolution/precipitation of minerals changes the porosity/permeability of reservoir and cap-rock. Thus, for adequate, safe and effective CO2 storage, the subsurface system needs to be fully understood. An important aspect for subsurface storage of CO2 is purity of this gas, which influences risk and cost of the process. In order to investigate the effects of CO2 plus impurities in a real case example, we have carried out medium-term (30 days) laboratory experiments (300 bars, 100° C) on reservoir and cap-rock core samples from gas fields in northeast Netherlands. In addition, we attempted to determine the maximum allowable concentration of one of the possible impurities in the CO2 stream (H2S) in these fields.
- The injected gases were CO2+100 ppm H2S and CO2+5000 ppm H2S were reacting with core samples and brine (Table 2.2). Before and after the experiments the core samples were analyzed by scanning electron microscope (SEM) and X-ray diffraction (XRD) for mineralogical variations. Also the permeability of the samples was measured. Following the experiments, dissolution of feldspars, carbonates and kaolinite was observed as expected. In addition, we observed fresh precipitation of kaolinite. However, two significant results were obtained when adding H2S in the CO2 stream. Firstly we observed precipitation of sulfate minerals (anhydrite and pyrite). This differs from results after pure CO2 injection where dissolution of anhydrite was dominant in the samples. Secondly, severe salt precipitation took place in the presence of H2S. This is caused mainly by the nucleation of anhydrite and pyrite which enabled halite precipitation and to a lesser degree by the higher solubility of H2S in water and higher water content of the gas phase in the presence of H2S. This was confirmed by modeling using CMG-GEM (CMG, 2011) modeling software.
- The precipitation of halite, anhydrite and pyrite affects the permeability of the samples in different ways. After CO2+100 ppm H2S injection, permeability of the reservoir samples increased by ≤3%. In caprock samples permeability increased by a 1.3. However, after addition of 5000 ppm H2S the permeability of all samples decreased significantly. In the case of CO2+100 ppm H2S, halite, anhydrite and pyrite precipitation did balance mineral dissolution, causing minimal variation in the permeability of samples.