Modeling of Non-Equilibrium Interphase Mass Transfer during Solvent-Aided Thermal Recovery Processes
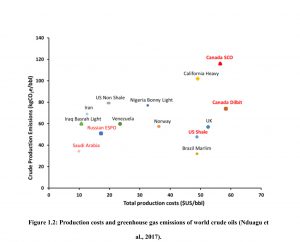
随着全球对能源需求的持续增长,重油和沥青储量的开采变得尤为重要。传统的重油开采方法由于油质过于粘稠而无法有效实施,因此需要采用增强型采油(EOR)方法,主要通过热或稀释来实现。蒸汽辅助重力泄油(SAGD)和循环蒸汽吞吐(CSS)是两种最具商业可行性的热采方法。这些方法通过蒸汽加热储层,降低沥青的粘度,使其流动。尽管这些热采过程取得了商业上的成功,但溶剂辅助热采过程因其在能源效率、环境影响和经济可行性方面的潜在优势而越来越受到工业界的关注。在溶剂辅助热采过程中,溶剂与蒸汽一起注入,以进一步通过质量和热传递以及溶剂向沥青中的扩散来降低沥青的粘度。
本研究的第一部分旨在解决溶剂溶解沥青的机制,特别是由于溶剂扩散而定义的相间传质系数。结果表明,较轻溶剂向沥青中的扩散低于较重溶剂,特别是在低温下。此外,发现较轻溶剂(如甲烷、乙烷和丙烷)的扩散主导相间传质系数相对较高。因此,对于设计和实施成功的溶剂辅助热采过程,非平衡相际传质现象的建模对较轻的溶剂更为重要。
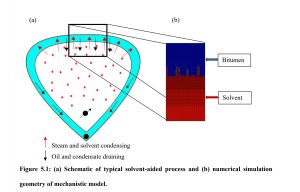
文章中还讨论了在当前油藏模拟模型中通常假设局部平衡,即模拟网格块瞬间达到平衡。然而,在实际中,这种假设在较大尺度或流速与质量和热传递相比较大的情况下常常失败。本研究的第二部分使用CMG-STARS模拟了丙烷辅助的沥青重力泄油过程,模型中包含了非平衡传质现象,使用动力学方法来模拟这一过程。结果表明,局部瞬时平衡的假设会导致典型油田规模模型的石油采收率降低3%至6%。这种差异可以通过包含非平衡相间传质来缓解。为丙烷/沥青混合物开发了非平衡相间传质系数的相关性,这些相关性可以作为模拟油田规模溶剂EOR过程非平衡相际传质的指南。
CMG软件应用情况
在本研究中,使用CMG-STARS油藏模拟器模拟丙烷辅助的沥青重力泄油过程。通过在模型中包含非平衡传质现象,研究者能够模拟这一过程,并使用动力学方法。结果表明,包含非平衡传质可以提高油藏模拟的准确性,并为油田规模的溶剂辅助热采过程提供了有价值的指导。
Abstract
As most of the heavy oil reserves in the world are too viscous to be exploited conventionally, enhanced oil recovery (EOR) methods are applied mainly through utilizing heat or dilution. Two thermal recovery methods stand out to be the most viable and commercially practical for exploiting extra-heavy and highly viscous oil reservoirs are Steam-Assisted Gravity Drainage (SAGD), and Cyclic Steam Stimulation (CSS) processes. Both processes apply heat to the reservoir using steam to reduce the viscosity of the bitumen rendering it mobile. Despite the commercial success of these thermal recovery processes, solvent-aided thermal recovery processes recently gained increased industrial interest for their potential to achieve higher energy efficiency, reduced environmental impact, and increased economic viability. In solvent-aided thermal processes, solvent is coinjected with steam to further aid in reducing bitumen viscosity through mass and heat transfer and diffusion of solvent into bitumen.
Several field trials of solvent-based recovery processes have been carried out and field results were mixed or inconclusive, and that can be attributed to the lack of knowledge of the physics and interrelated mechanisms involved with interphase-mass transfer and solvent dissolution into bitumen. The first part of this thesis aims to address the mechanisms of solvent dissolution into bitumen due to solvent diffusion and defines the key parameter of diffusive dominant interphase-mass transfer coefficient for several solvent/bitumen binary mixtures. The results show that the diffusion of lighter solvents into bitumen is lower than heavier solvents particularly at low temperatures. Also, it was found that the diffusion dominant interphase mass transfer coefficient is relatively higher for lighter solvents such as methane, ethane, and propane. Therefore, modelling of the non-equilibrium interphase mass transfer phenomena is relatively more important for lighter solvents for designing and implementing a successful solvent-aided thermal recovery process.
One of the most important mechanisms involved in solvent-aided thermal recovery processes is interphase-mass transfer phenomena which involves a variation of a system property due to a non-equilibrium state. However, in current reservoir simulation models a local equilibrium is assumed such that a simulation grid block is at instantaneous equilibrium. In reality, local equilibrium assumption often fails at larger scales or in situations where flow velocities are large compared to that of mass or heat transfer. In the second part of this thesis, solvent-aided gravity drainage of bitumen was simulated with propane as a solvent using CMG-STARS. The effect of non-equilibrium mass transfer was included in the model to simulate the process using a kinetic approach. The results show that the assumption of the local instantaneous equilibrium result in 3% to 6% lower oil recovery for the typical field scale simulation models. This difference in oil recovery can be mitigated fairly through the inclusion of the non-equilibrium interphase mass transfer. Correlations for the non-equilibrium interphase mass transfer coefficients for propane/bitumen mixture were developed which can be used as guidelines for modelling the non-equilibrium interphase mass transfer for field scale simulations of solvent-based EOR processes.
作者单位
Abdullah Al-Gawfi 加拿大卡尔加里大学(University of Calgary) 化学与石油工程研究生项目