Impact of rock mineralogy on reactive transport of CO2 during carbon sequestration in a saline aquifer
本文通过数值模拟研究了深部咸水层中岩石矿物组成对CO₂地质封存过程中反应性运移的影响。研究表明,不同矿物(如石英、方解石、钙长石等)通过溶解-沉淀反应显著影响CO₂的封存机制。方解石在低pH下初期溶解,随后在约300年后沉淀;钙长石溶解为高岭石沉淀提供离子,其释放的Ca²⁺促进碳酸盐矿物沉淀。孔隙度变化早期由方解石溶解主导,后期与矿物沉淀相关。敏感性分析表明,钙长石和伊利石对CO₂矿化封存(相关系数0.68和0.46)起关键作用,而方解石和高岭石则更影响溶解度封存。矿化度升高会抑制CO₂溶解,限制其参与地球化学反应。
CMG软件解决方案
- 模型构建
- 使用CMG-GEM组分模拟器建立三维均质模型,网格尺寸7000m×7000m×250m,划分为20×20×5网格单元。
- 注入井以恒定速度(1.4×10⁶标准m³/天)注入超临界CO₂,持续25年,随后关闭模拟3000年。
- 考虑CO₂扩散、溶解及与岩石矿物的地球化学反应,集成Peng-Robinson状态方程(EOS)描述流体相态。
- 化学反应模型
- 包含7种矿物(方解石、钙长石、高岭石等)的动力学反应及3种水相平衡反应(如CO₂水合生成碳酸)。
- 使用Debye-Hückel模型计算活度系数,矿物反应速率基于表面反应动力学(如钙长石溶解速率参数来自Ague & Brimhall, 1989)。
- 物性参数
- 初始孔隙度38%、渗透率2000mD,盐水矿化度3万ppm。
- 相对渗透率曲线基于高渗透砂岩典型数据,孔隙度变化通过Kozeny-Carman方程关联渗透率。
模拟结果
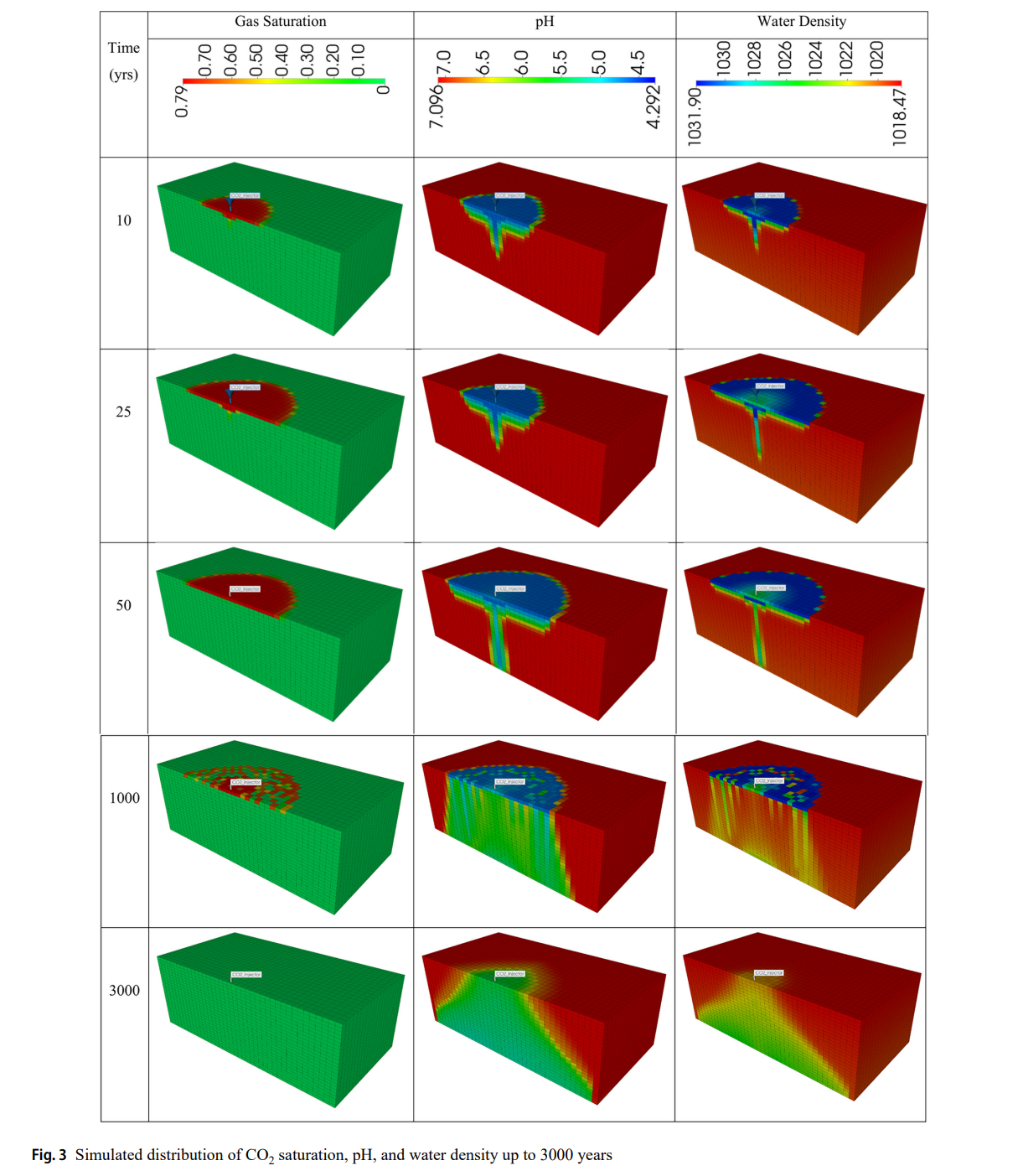
- CO₂迁移与反应
- CO₂羽流因浮力向上迁移,溶解后形成高密度盐水下沉,引发自然对流。
- pH最低值4.29出现在注入区,随时间逐渐回升(3000年后达5.3)。
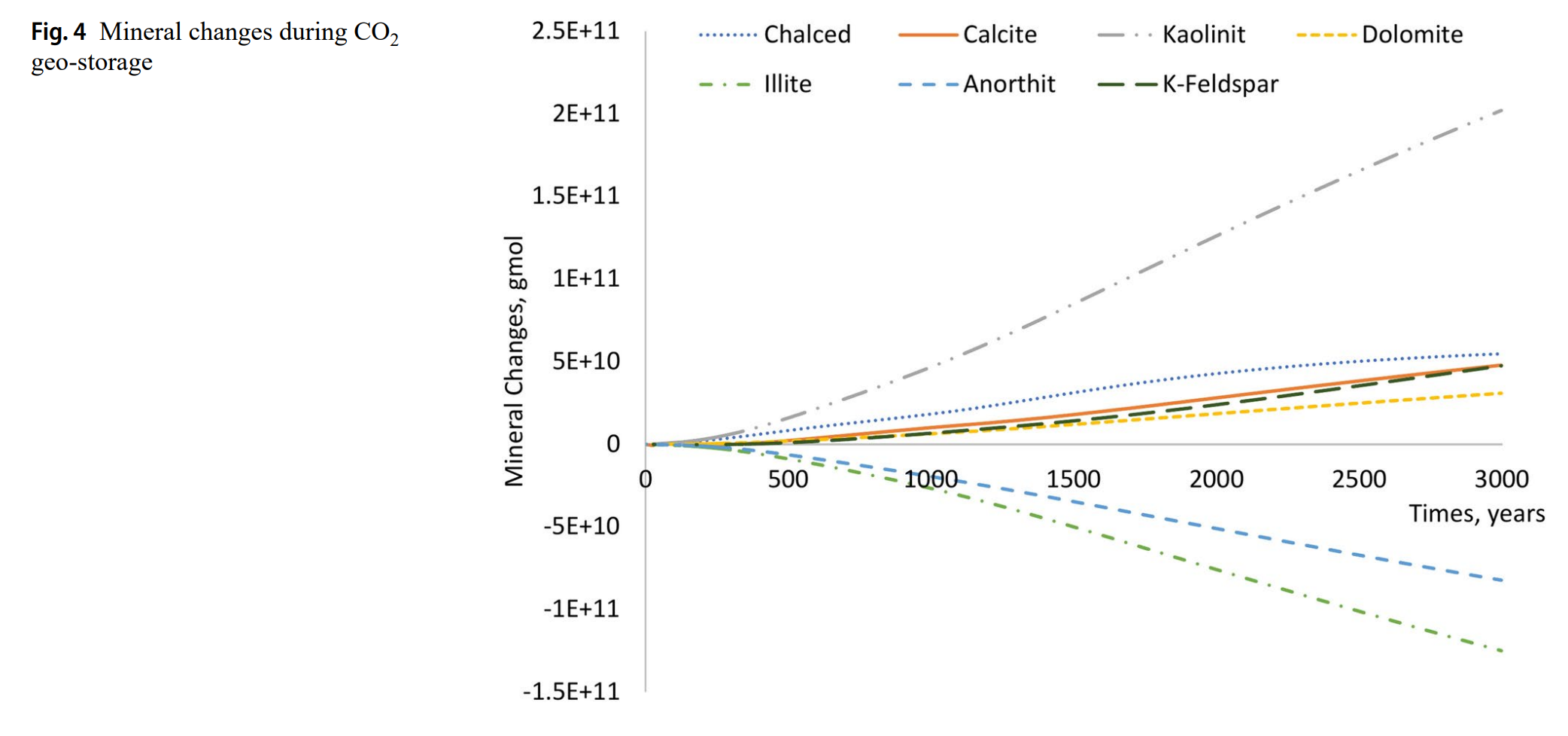
- 矿物动态
- 方解石初期溶解增加孔隙度(+0.2%),后期沉淀导致孔隙度下降。
- 钙长石持续溶解,伊利石快速溶解,高岭石显著沉淀(提供Al³⁺和SiO₂)。
- 封存机制
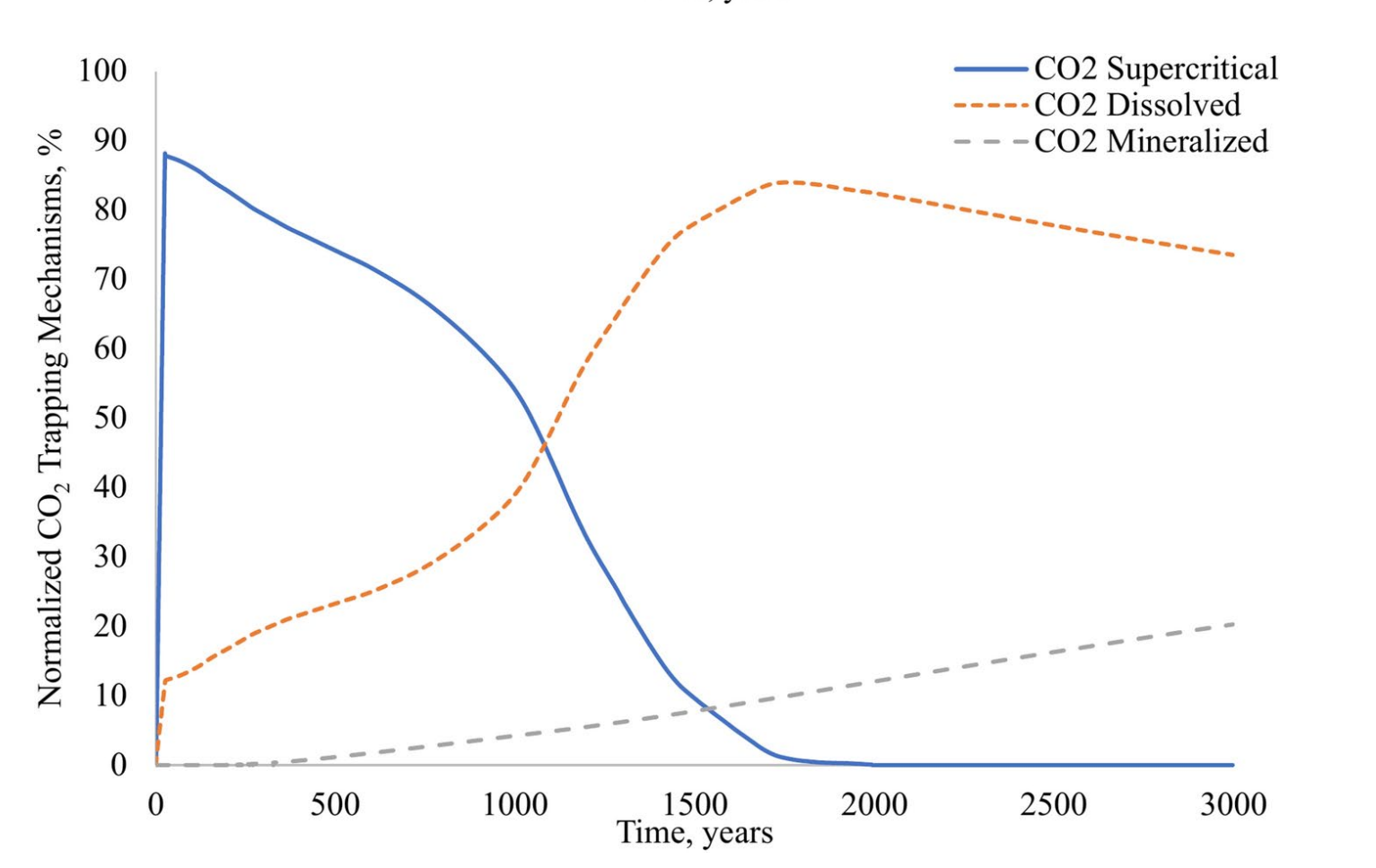
- 注入期:结构封存(88%)和溶解封存(12%)主导。
- 封存后期:溶解封存占比升至84%,矿化封存300年后启动,3000年达20%。
- 敏感性分析
- 钙长石浓度≥0.05时矿化封存效率饱和,盐度>60Mppm显著抑制溶解封存。
结论
- 矿物组成(尤其钙长石和伊利石)是CO₂矿化封存的关键驱动因素。
- 高盐度(>60Mppm)通过限制CO₂溶解降低矿化效率。
- 碳酸盐岩地层(方解石+白云石)矿化能力弱于含钙长石的砂岩。
Abstract
Deep saline aquifers offer a vast long-term storage capacity for CO2. The diverse mineral compositions in CO2 geological storage systems complicate the reactive transport of CO2. Despite extensive research on carbon sequestration in saline aquifers, the impact of rock mineralogy on the CO2 trapping mechanisms needs further investigation. This study addresses this gap by employing numerical simulations to investigate how different minerals affect the reactive transport of CO2 during injection into saline aquifers. A compositional simulation model was created, wherein CO2 is injected for 25 years, followed by a 3000-year shut-in period. Various parameters, including CO2 plume migration, changes in pH, water density variations, mineral dissolution and precipitation, and porosity changes in the reservoir rock, are examined to understand the complex CO2–brine–rock interactions. The effect of formation mineralogy on CO2 trapping mechanisms was investigated. To do so, a sensitivity study was conducted and then, special cases with extreme mineral concentrations were studied. The simulation results highlight the profound effects of different minerals on the CO2 trapping mechanisms. Quartz exhibits minimal dissolution, while calcite plays a crucial role, initially dissolving due to low pH and later precipitating after around 300 years. Anorthite dissolution provides ions for kaolinite precipitation, and the release of calcium ions from anorthite dissolution contributes to carbonate minerals precipitation. Porosity changes in the reservoir rock are observed, with regions experiencing an increase due to early calcite dissolution and subsequent changes associated with mineral precipitation. Sensitivity analysis showed that Anorthite and Illite facilitate more CO2 mineralization, with Anorthite and Illite showing 0.68 and 0.46 correlation with mineral trapping, respectively. Conversely, minerals like Calcite and Kaolinite positively correlated with CO2 solubility, with correlations of + 0.21 and + 0.23 respectively, without significantly promoting its conversion into mineral forms, while Quartz, K-Feldspar, and Dolomite show minor effects on CO2 trapping mechanisms. Elevating Anorthite concentrations speeds up CO2 mineralization, ensuring secure storage in saline aquifers, with a minimum threshold concentration of 0.05 volume fraction; beyond this point, no further changes in CO2 mineralization are observed. Formation brine salinity significantly affects CO2 solubility and, consequently, mineral trapping, as higher salinity levels hinder CO2 dissolution and restrict its availability for geochemical reactions with rock minerals. This study offers insights that can inform reservoir characterization and management practices, ultimately enhancing the effectiveness of carbon sequestration efforts.
作者单位
沙特阿拉伯法赫德国王石油与矿产大学(KFUPM)
