摘要:本研究考察了将二氧化碳注入深层咸水层时的影响,考虑了蓝色氢生产中存在的杂质。设计了一个考虑3.5%和20%杂质浓度的储层条件的流体模型。结果表明,甲烷在3.5%和20%时导致密度降低95.16%和76.16%,而硫化氢导致降低99.56%和98.77%。粘度随着甲烷含量增加至20%而从0.045降至0.037 cp;然而,硫化氢并不会影响粘度。值得注意的是,含硫化氢的二氧化碳对这些属性的影响比甲烷小。我们的模拟模型基于Gorae-V属性,对注入进行了10年的模拟,然后进行了100年的监测。与纯二氧化碳注入相比,甲烷在3.5%时在八年零十一个月达到最大压力,在20%时在八年达到最大压力;而硫化氢在三年零两个月和九年六个月后分别达到最大压力。这些时间影响了注入的二氧化碳量。含甲烷的情况下,注入效率降低了73.16%,而含硫化氢时则降低了81.99%。分析二氧化碳在溶解和残余困陷中的存储效率,以研究杂质的影响。残余陷阱的效率始终随着甲烷而下降,但与硫化氢则会上升。在20%浓度下,甲烷陷具有更高的效率,并在注入结束时硫化氢在监测端点拥有更高的效率。在碳捕集和存储项目中,甲烷杂质需要去除,而硫化氢可能不需要脱硫,因为其对二氧化碳存储效率的影响很小。因此,将碳捕集与存储(CCS)应用于含硫化氢作为杂质的二氧化碳排放可能通过降低额外成本实现经济可行的运营。
关键词:二氧化碳注入,二氧化碳存储,杂质,气储层,碳捕集与存储
Abstract
In this study, we examined the effect of CO2 injection into deep saline aquifers, considering impurities present in blue hydrogen production. A fluid model was designed for reservoir conditions with impurity concentrations of 3.5 and 20%. The results showed that methane caused density decreases of 95.16 and 76.16% at 3.5 and 20%, respectively, whereas H2S caused decreases of 99.56 and 98.77%, respectively. Viscosity decreased from 0.045 to 0.037 cp with increasing methane content up to 20%; however, H2S did not affect the viscosity. Notably, CO2 with H2S impacted these properties less than methane. Our simulation model was based on the Gorae-V properties and simulated injections for 10 years, followed by 100 years of monitoring. Compared with the pure CO2 injection, methane reached its maximum pressure after eight years and eleven months at 3.5% and eight years at 20%, whereas H2S reached maximum pressure after nine years and two months and nine years and six months, respectively. These timings affected the amount of CO2 injected. With methane as an impurity, injection efficiency decreased up to 73.16%, whereas with H2S, it decreased up to 81.99% with increasing impurity concentration. The efficiency of CO2 storage in the dissolution and residual traps was analyzed to examine the impact of impurities. The residual trap efficiency consistently decreased with methane but increased with H2S. At 20% concentration, the methane trap exhibited higher efficiency at the end of injection; however, H2S had a higher efficiency at the monitoring endpoint. In carbon capture and storage projects, methane impurities require removal, whereas H2S may not necessitate desulfurization due to its minimal impact on CO2 storage efficiency. Thus, the application of carbon capture and storage (CCS) to CO2 emissions containing H2S as an impurity may enable economically viable operations by reducing additional costs.
Keywords:
carbon capture and storage; impurities; hydrogen production; saline aquifer
Conclusions
In this study, CO2 storage efficiency was analyzed when CO2, along with impurities such as methane and H2S, which are captured by CO2 in the hydrogen production process, was injected into a deep saline aquifer. Fluid modeling was conducted by mixing methane and H2S with CO2, followed by the simulation of injection into a 3D saline aquifer model. The following conclusions were drawn from this study:
1. Impurities affected the CO2 stream under initial reservoir conditions. In the case of methane, the density decreases compared to that of pure CO2, and its volume ratio increases, whereas the viscosity decreases. In contrast, a smaller change in density is shown in H2S than in methane, and its volume decreases relative to the injection concentration, whereas, compared to pure CO2, its viscosity increases;
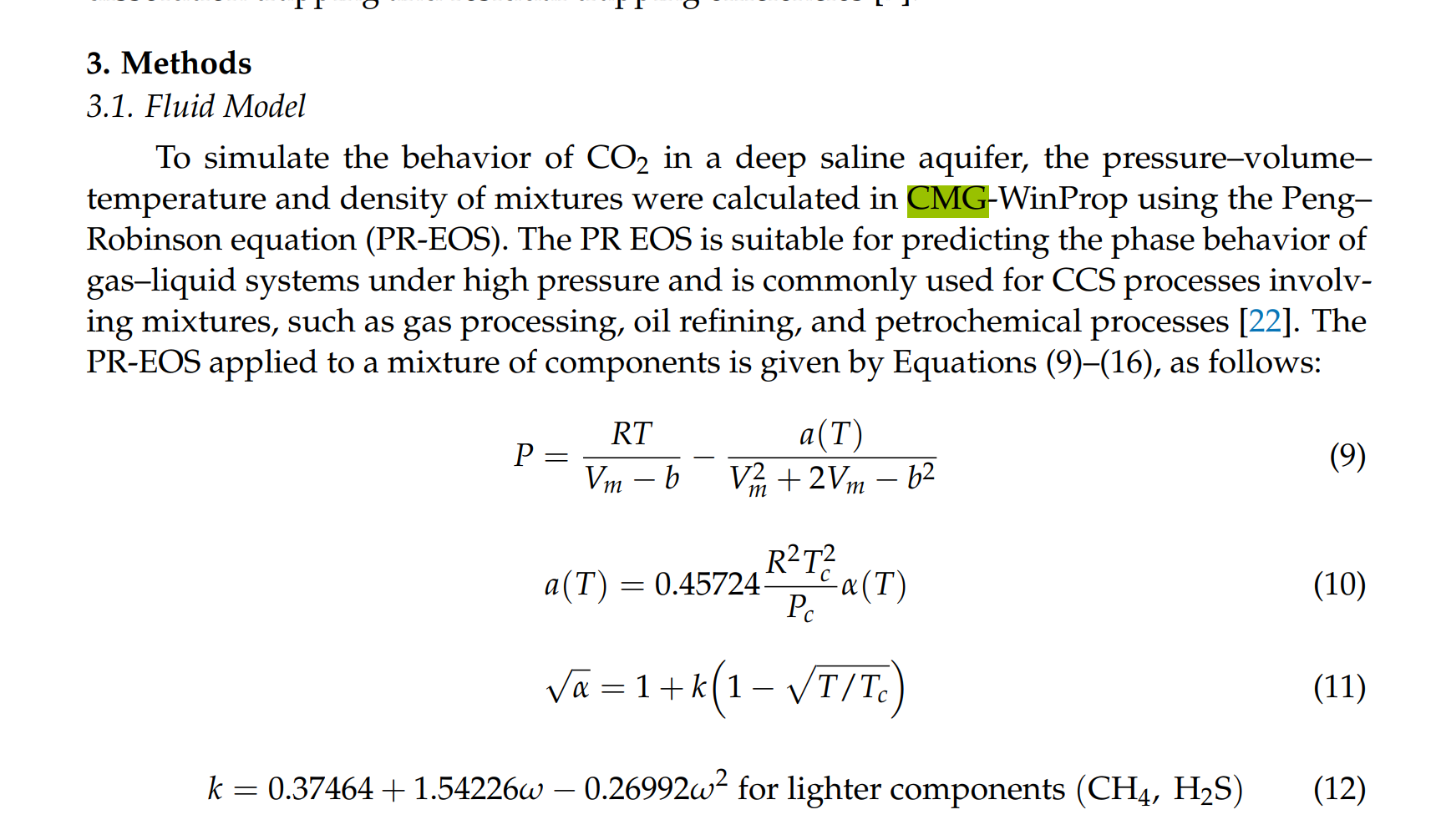
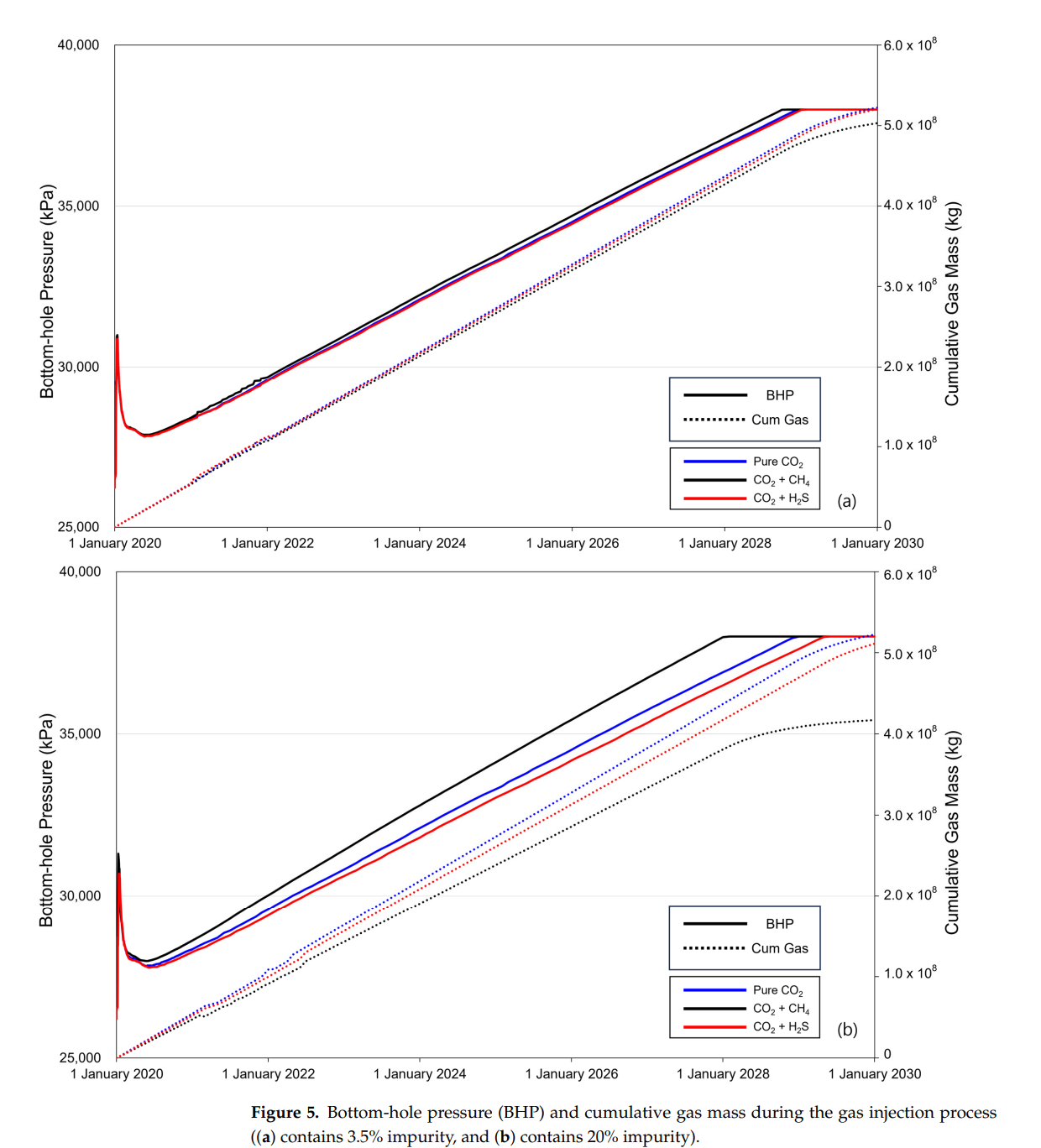
2. When the same amount of gas is injected into the reservoir, reaching the pressure limit of the reservoir occurs more rapidly when methane is present, while that with H2S takes longer than that with pure CO2. The difference in the time required to reach the pressure limit increases with impurity concentration. These phenomena affect the amount of CO2 injected into the stream;
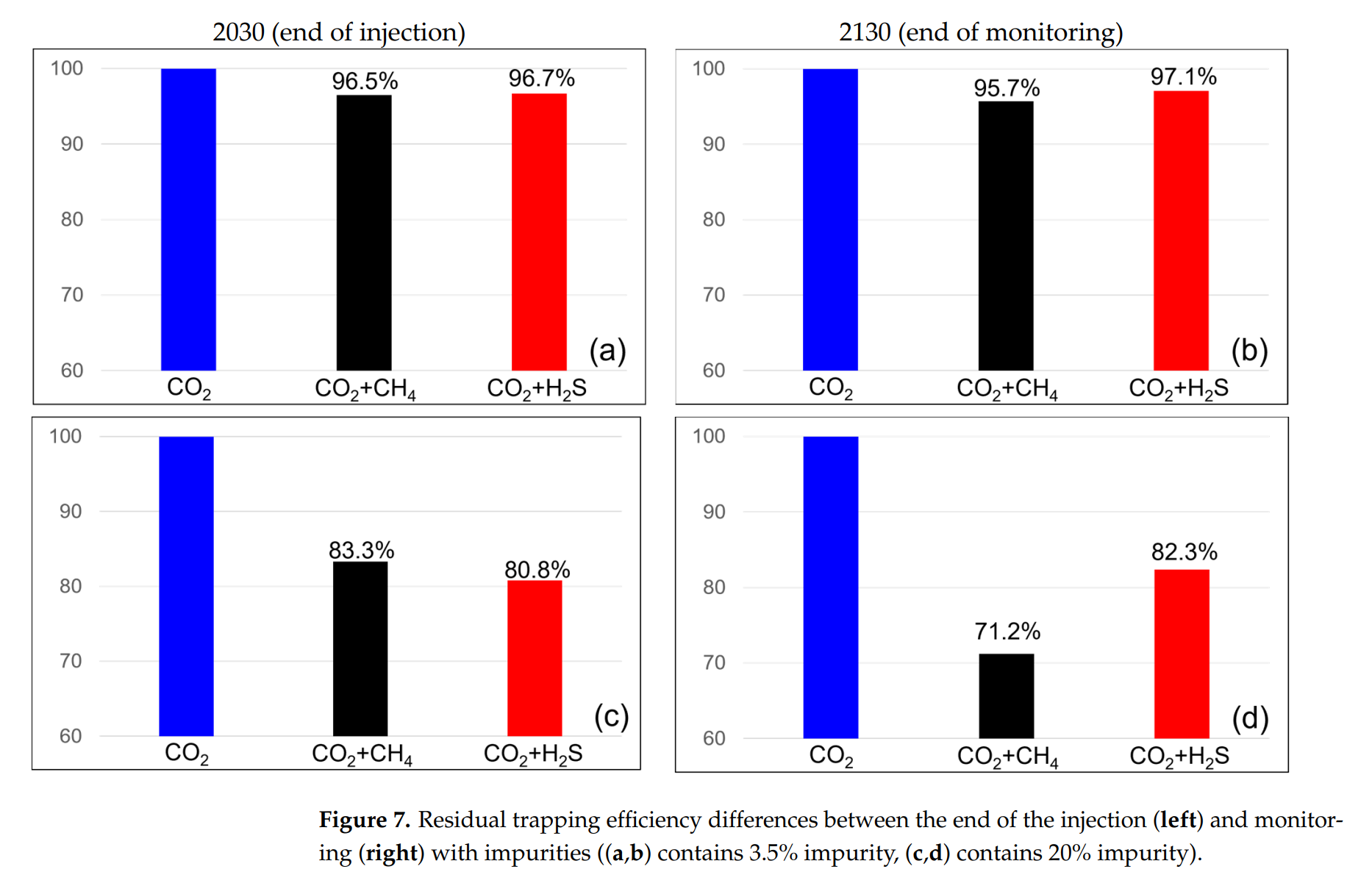
3. Once the pressure limit is reached, the injection amounts differ, leading to variations in CO2 storage. Methane present at 3.5% resulted in 95.04% CO2 injection, and at 20%, only 73.16% CO2 was injected. In contrast, with H2S at 3.5%, 96.91% CO2 was injected, and at 20%, 81.99% CO2 was injected. Consequently, methane stores less CO2 relative to the mixture ratio, whereas H2S stores more;
4. Methane results in a relatively low density, reducing the contact time between the brine and CO2 and decreasing the dissolution effect. The CO2 volume ratio within the gas mixture also decreases, leading to a reduced amount of CO2 that can be retained in the rock pores;
5. When storing CO2 underground, methane causes a rapid increase in BHP and decreases storage efficiency. However, if H2S is not removed and injection occurs with CO2, the BHP increases more slowly than with the pure CO2 injection. Additionally, the volume ratio of CO2 in the mixed gas decreases, leading to improved storage efficiency. For CCS projects, methane impurities in the CO2 stream require removal. In contrast, H2S may not require steps such as desulfurization because it does not significantly impact CO2 storage efficiency. Employing CCS for carbon dioxide emissions containing H2S as an impurity can enable economically viable operations by reducing additional costs.
