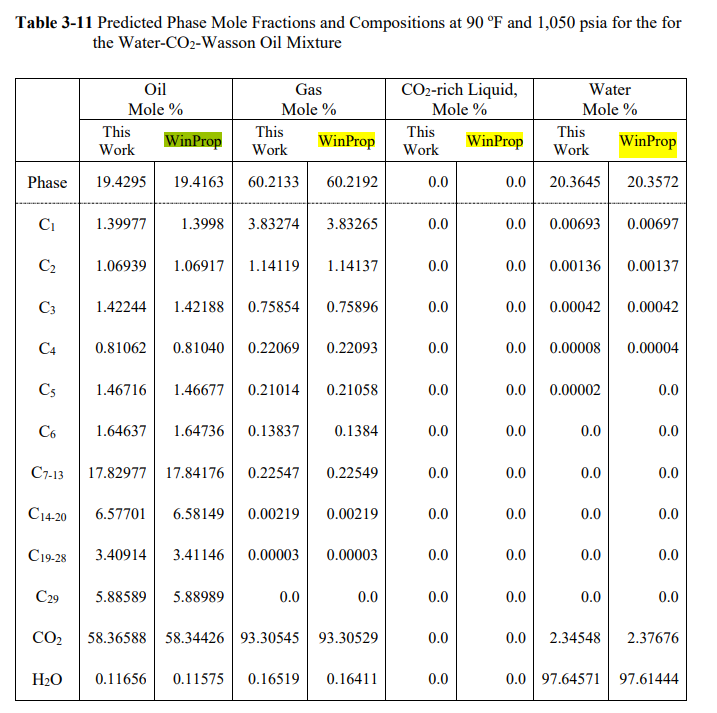
Modelling Water-Hydrocarbon Mutual Solubility in Multiphase Equilibrium Calculations
自20世纪80年代以来,多相平衡计算得到了较好发展。通常情况下,计算中不包括水,尽管水中溶解的碳氢化合物和二氧化碳的数量可能很大。有几种已发表的四相闪蒸计算方法使用单个立方型状态方程来模拟碳氢化合物和水相,但根据状态方程模拟预测的气体在水中的溶解度比实验数据低几个数量级。
本文提出了一种广义多相闪蒸计算算法,以同时考虑CO2/原油体系的多相行为和水-烃互溶性。用立方型状态方程模拟了烃相,用亨利定律常数模拟了水相。我们的结果与实验数据和商业软件的计算结果进行了比较,以验证算法在不同类型的平衡中的有效性。
Abstract
Since the 1980s, multiphase equilibrium calculations have been well developed. Usually, water is excluded from the calculations although the amount of dissolved hydrocarbons and CO2 in water can be substantial. There are several published four-phase flash calculation methods using a single cubic EOS to model both hydrocarbon and aqueous phases, but the predicted gas solubility in water modeled from an EOS is orders of magnitude lower than experimental data. In this thesis, a generalized multiphase flash calculation algorithm is developed to address both the multiple phase behavior of a CO2/crude oil system and water-hydrocarbon mutual solubility simultaneously. The hydrocarbon phases are modeled with a cubic EOS, and the water phase is modelled with Henry’s law constants. Our results are compared with experimental data and calculation results from commercial software to validate the algorithm in different types of equilibria.